CEREBYX- fosphenytoin sodium injection, solution
CEREBYX by
Drug Labeling and Warnings
CEREBYX by is a Prescription medication manufactured, distributed, or labeled by Pfizer Laboratories Div Pfizer Inc, Pfizer Inc, Pharmacia & Upjohn Company LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CEREBYX® safely and effectively. See full prescribing information for CEREBYX®.
CEREBYX® (fosphenytoin sodium) injection, for intravenous or intramuscular use
Initial U.S. Approval: 1996WARNING: CARDIOVASCULAR RISK ASSOCIATED WITH RAPID INFUSION RATES
See full prescribing information for complete boxed warning.
- The rate of intravenous CEREBYX administration should not exceed 150 mg phenytoin sodium equivalents (PE) per minute in adults and 2 mg PE/kg/min (or 150 mg PE/min, whichever is slower) in pediatric patients because of the risk of severe hypotension and cardiac arrhythmias.
- Careful cardiac monitoring is needed during and after administering intravenous CEREBYX.
- Reduction in rate of administration or discontinuation of dosing may be needed (2.3, 2.4, 5.2).
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
CEREBYX is indicated for the treatment of generalized tonic-clonic status epilepticus and prevention and treatment of seizures occurring during neurosurgery. CEREBYX can also be substituted, as short-term use, for oral phenytoin. CEREBYX should be used only when oral phenytoin administration is not possible. (1)
DOSAGE AND ADMINISTRATION
- The dose, concentration, and infusion rate of CEREBYX should always be expressed as phenytoin sodium equivalents (PE) (2.1)
- For Status Epilepticus:
-
For Non-emergent Loading and Maintenance Dosing:
- Adult loading dose is 10 to 20 mg PE/kg given IV or IM; initial maintenance dose is 4 to 6 mg PE/kg/day in divided doses (2.4)
- Pediatric loading dose is 10 to 15 mg PE/kg at a rate of 1 to 2 mg PE/kg/min (or 150 mg PE/min, whichever is slower); initial maintenance dose is 2 to 4 mg PE/kg every 12 hours at a rate of 1 to 2 mg PE/kg/min (or 100 mg PE/min, whichever is slower) (2.4)
- Intramuscular Administration:
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Dosing Errors: Do not confuse the amount of drug to be given in PE with the concentration of the drug in the vial. Ensure the appropriate volume is withdrawn from the vial when preparing for administration. (5.1)
- Withdrawal Precipitated Seizure: May precipitate status epilepticus. Dose reductions or discontinuation should be done gradually. (5.3)
- Serious Dermatologic Reactions: Discontinue at the first sign of a rash, unless clearly not drug-related. If signs or symptoms suggest SJS/TEN, CEREBYX should not be resumed; consider alternative therapy. (5.4)
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity: If signs or symptoms of hypersensitivity are present, evaluate the patient immediately. Discontinue if an alternative etiology cannot be established. (5.5)
- Angioedema: Discontinue immediately if symptoms of angioedema such as facial, perioral, or upper airway swelling occur. (5.7)
- Hematopoietic Complications: If occurs, follow-up observation is indicated and an alternative antiepileptic treatment should be used. (5.9)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥10%) are:
- Adults: pruritus, nystagmus, dizziness, somnolence, and ataxia
- Pediatrics: vomiting, nystagmus, and ataxia (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer, Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: CARDIOVASCULAR RISK ASSOCIATED WITH RAPID INFUSION RATES
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions to Avoid Dosing Errors
2.2 Preparation
2.3 Status Epilepticus
2.4 Non-emergent Loading and Maintenance Dosing
2.5 Laboratory Tests and Monitoring Levels
2.6 Parenteral Substitution for Oral Phenytoin Therapy
2.7 Dosing in Patients with Renal or Hepatic Impairment or Hypoalbuminemia
2.8 Dosing in Geriatrics
2.9 Dosing during Pregnancy
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Dosing Errors
5.2 Cardiovascular Risk Associated with Rapid Infusion
5.3 Withdrawal Precipitated Seizure, Status Epilepticus
5.4 Serious Dermatologic Reactions
5.5 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity
5.6 Hypersensitivity
5.7 Angioedema
5.8 Hepatic Injury
5.9 Hematopoietic Complications
5.10 Sensory Disturbances
5.11 Local Toxicity (Including Purple Glove Syndrome)
5.12 Phosphate Load
5.13 Renal or Hepatic Disease or Hypoalbuminemia
5.14 Exacerbation of Porphyria
5.15 Teratogenicity and Other Harm to the Newborn
5.16 Slow Metabolizers of Phenytoin
5.17 Hyperglycemia
5.18 Serum Phenytoin Levels above Therapeutic Range
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Drugs that Affect Phenytoin or CEREBYX
7.2 Drugs Affected by Phenytoin or CEREBYX
7.3 Drug/Laboratory Test Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal and/or Hepatic Impairment, or Hypoalbuminemia
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: CARDIOVASCULAR RISK ASSOCIATED WITH RAPID INFUSION RATES
The rate of intravenous CEREBYX administration should not exceed 150 mg phenytoin sodium equivalents (PE) per minute in adults and 2 mg PE/kg/min (or 150 mg PE/min, whichever is slower) in pediatric patients because of the risk of severe hypotension and cardiac arrhythmias. Careful cardiac monitoring is needed during and after administering intravenous CEREBYX. Although the risk of cardiovascular toxicity increases with infusion rates above the recommended infusion rate, these events have also been reported at or below the recommended infusion rate. Reduction in rate of administration or discontinuation of dosing may be needed [see Dosage and Administration (2.3, 2.4) and Warnings and Precautions (5.2)].
-
1 INDICATIONS AND USAGE
CEREBYX is indicated for the treatment of generalized tonic-clonic status epilepticus and prevention and treatment of seizures occurring during neurosurgery. CEREBYX can also be substituted, short-term, for oral phenytoin. CEREBYX should be used only when oral phenytoin administration is not possible [see Dosage and Administration (2.4) and Warnings and Precautions (5.2)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions to Avoid Dosing Errors
Use caution when administering CEREBYX because of the risk of dosing errors [see Warnings and Precautions (5.1)].
Phenytoin Sodium Equivalents (PE)
The dose, concentration, and infusion rate of CEREBYX should always be expressed as phenytoin sodium equivalents (PE). There is no need to perform molecular weight-based adjustments when converting between fosphenytoin and phenytoin sodium doses. CEREBYX should always be prescribed and dispensed in phenytoin sodium equivalent units (PE). The amount and concentration of fosphenytoin is always expressed in terms of mg of phenytoin sodium equivalents (mg PE).
Concentration of 50 mg PE/mL
Do not confuse the concentration of CEREBYX with the total amount of drug in the vial.
Errors, including fatal overdoses, have occurred when the concentration of the vial (50 mg PE/mL) was misinterpreted to mean that the total content of the vial was 50 mg PE. These errors have resulted in two- or ten-fold overdoses of CEREBYX since each of the vials actually contains a total of 100 mg PE (2 mL vial) or 500 mg PE (10 mL vial). Ensure the appropriate volume of CEREBYX is withdrawn from the vial when preparing the dose for administration. Attention to these details may prevent some CEREBYX medication errors from occurring.
2.2 Preparation
Prior to intravenous (IV) infusion, dilute CEREBYX in 5% dextrose or 0.9% saline solution for injection to a concentration ranging from 1.5 to 25 mg PE/mL. The maximum concentration of CEREBYX in any solution should be 25 mg PE/mL. When CEREBYX is given as an IV infusion, CEREBYX needs to be diluted and should only be administered at a rate not exceeding 150 mg PE/min.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
For single-dose only. After opening, any unused product should be discarded.
2.3 Status Epilepticus
- Because of the risk of hypotension and cardiac arrhythmias, the rate of administration for IV CEREBYX should be no greater than 150 mg PE/min in adults and 2 mg PE/kg/min (or 150 mg PE/min, whichever is slower) in pediatric patients [see Warnings and Precautions (5.2)]. Continuous monitoring of the electrocardiogram, blood pressure, and respiratory function is essential, and the patient should be observed throughout the period where maximal serum phenytoin concentrations occur, approximately 10 to 20 minutes after the end of CEREBYX infusions.
- Because the full antiepileptic effect of phenytoin, whether given as CEREBYX or parenteral phenytoin, is not immediate, other measures, including concomitant administration of an IV benzodiazepine, will usually be necessary for the control of status epilepticus.
- The loading dose should be followed by maintenance doses of either CEREBYX or phenytoin [see Dosage and Administration (2.4)].
- If administration of CEREBYX does not terminate seizures, the use of other anticonvulsants and other appropriate measures should be considered.
Adult and Pediatric Status Epilepticus Dosing:
Table 1. Status Epilepticus Loading Dosages Population Dosage Infusion rate Adult 15 mg PE/kg to 20 mg PE/kg 100 mg PE/min to 150 mg PE/min, do not exceed a maximum rate of 150 mg PE/min Pediatric (Birth to less than 17 years of age) 15 mg PE/kg to 20 mg PE/kg 2 mg PE/kg/min, or 150 mg PE/min, whichever is slower Even though loading doses of CEREBYX have been given by the IM route for other indications when IV access is impossible, IM CEREBYX should ordinarily not be used in the treatment of status epilepticus because therapeutic phenytoin concentrations may not be reached as quickly as with IV administration.
Intramuscular administration of CEREBYX should ordinarily not be used in pediatric patients. When IV access has been impossible, loading doses of CEREBYX have been given by the IM route.
2.4 Non-emergent Loading and Maintenance Dosing
- Because of the risk of hypotension and cardiac arrhythmias, the rate of administration for IV CEREBYX should be no greater than 150 mg PE/min in adults. For loading doses in pediatric patients, the rate should not exceed 2 mg PE/kg/min (or 150 mg PE/min, whichever is slower). For maintenance doses in pediatric patients, the rate should not exceed 1 to 2 mg PE/kg/min (or 100 mg PE/min, whichever is slower). Continuous monitoring of the electrocardiogram, blood pressure, and respiratory function is essential, and the patient should be observed throughout the period where maximal serum phenytoin concentrations occur (approximately 10 to 20 minutes after the end of CEREBYX infusions).
- After the initial maintenance dose, subsequent maintenance doses should be individualized by monitoring serum phenytoin concentrations to achieve a target therapeutic concentration of phenytoin [see Dosage and Administration (2.5) and Warnings and Precautions (5.18)].
Adult and Pediatric Non-emergent Loading and Maintenance Dosing:
Table 2. Non-emergent Loading Dosages Population Dosage Infusion rate Adult 10 mg PE/kg to 20 mg PE/kg Not to exceed a maximum rate of 150 mg PE/min Pediatric (Birth to less than 17 years of age) 10 mg PE/kg to 15 mg PE/kg 1 mg PE/kg/min to 2 mg PE/kg/min, or 150 mg PE/min, whichever is slower Table 3. Maintenance Dosages Population Dosage Infusion rate Adult Initial Maintenance Dosage: 4 mg PE/kg/day to 6 mg PE/kg/day in divided doses Not to exceed a maximum rate of 150 mg PE/min Pediatric (Birth to less than 17 years of age) Initial Maintenance Dosage: 2 mg PE/kg to 4 mg PE/kg (dose given 12 hours after the loading dose) 1 mg PE/kg/min to 2 mg PE/kg/min, or 100 mg PE/min, whichever is slower Maintenance Dosage after Initial Maintenance Dosage: 4 mg PE/kg/day to 8 mg PE/kg/day in divided doses (continued every 12 hours after initial maintenance dose) 1 mg PE/kg/min to 2 mg PE/kg/min, or 100 mg PE/min, whichever is slower Because of the risks of cardiac and local toxicity associated with intravenous CEREBYX, oral phenytoin should be used whenever possible. Intramuscular administration of CEREBYX should ordinarily not be used in pediatric patients.
2.5 Laboratory Tests and Monitoring Levels
Laboratory Tests:
CEREBYX (or phenytoin) doses are usually selected to attain therapeutic serum total phenytoin concentrations of 10 to 20 mcg/mL (unbound phenytoin concentrations of 1 to 2 mcg/mL). Following CEREBYX administration, it is recommended that phenytoin concentrations not be monitored until conversion to phenytoin is essentially complete. This occurs within approximately 2 hours after the end of IV infusion and 4 hours after intramuscular (IM) injection. Prior to complete conversion, commonly used immunoanalytical techniques, such as TDx®/TDxFLx™ (fluorescence polarization) and Emit® 2000 (enzyme multiplied), may significantly overestimate serum phenytoin concentrations because of cross-reactivity with fosphenytoin. The error is dependent on serum phenytoin and fosphenytoin concentration (influenced by CEREBYX dose, route and rate of administration, and time of sampling relative to dosing), and analytical method. Chromatographic assay methods accurately quantitate phenytoin concentrations in biological fluids in the presence of fosphenytoin. Prior to complete conversion, blood samples for phenytoin monitoring should be collected in tubes containing EDTA as an anticoagulant to minimize ex vivo conversion of fosphenytoin to phenytoin. However, even with specific assay methods, phenytoin concentrations measured before conversion of fosphenytoin is complete will not reflect phenytoin concentrations ultimately achieved.
Monitoring Levels:
Trough levels provide information about clinically effective serum level range and are obtained just prior to the patient's next scheduled dose. Peak levels indicate an individual's threshold for emergence of dose-related side effects and are obtained at the time of expected peak concentration. Therapeutic effect without clinical signs of toxicity occurs more often with serum total phenytoin concentrations between 10 and 20 mcg/mL (unbound phenytoin concentrations of 1 to 2 mcg/mL), although some mild cases of tonic-clonic (grand mal) epilepsy may be controlled with lower serum levels of phenytoin. In patients with renal or hepatic disease, or in those with hypoalbuminemia, the monitoring of unbound phenytoin concentrations may be more relevant [see Dosage and Administration (2.7)].
2.6 Parenteral Substitution for Oral Phenytoin Therapy
When treatment with oral phenytoin is not possible, CEREBYX can be substituted for oral phenytoin at the same total daily phenytoin sodium equivalents (PE) dose. Dilantin capsules are approximately 90% bioavailable by the oral route. Phenytoin, derived from administration of CEREBYX, is 100% bioavailable by both the IM and IV routes. For this reason, serum phenytoin concentrations may increase modestly when IM or IV CEREBYX is substituted for oral phenytoin sodium therapy. The rate of administration for IV CEREBYX should be no greater than 150 mg PE/min in adults and 2 mg PE/kg/min (or 150 mg PE/min, whichever is slower) in pediatric patients. In controlled trials, IM CEREBYX was administered as a single daily dose utilizing either 1 or 2 injection sites. Some patients may require more frequent dosing. Intramuscular administration of CEREBYX should ordinarily not be used in pediatric patients.
2.7 Dosing in Patients with Renal or Hepatic Impairment or Hypoalbuminemia
Because the fraction of unbound phenytoin (the active metabolite of CEREBYX) is increased in patients with renal or hepatic disease, or in those with hypoalbuminemia, the monitoring of phenytoin serum levels should be based on the unbound fraction in those patients. After IV CEREBYX administration to patients with renal and/or hepatic disease, or in those with hypoalbuminemia, fosphenytoin clearance to phenytoin may be increased without a similar increase in phenytoin clearance. This has the potential to increase the frequency and severity of adverse events [see Warnings and Precautions (5.13)].
2.8 Dosing in Geriatrics
The clearance of phenytoin (the active metabolite of CEREBYX) is decreased slightly in elderly patients and lower or less frequent dosing may be required [see Clinical Pharmacology (12.3)].
2.9 Dosing during Pregnancy
Decreased serum concentrations of phenytoin (the active metabolite of CEREBYX) may occur during pregnancy because of altered phenytoin pharmacokinetics [see Clinical Pharmacology (12.3)]. Periodic measurement of serum phenytoin concentrations should be performed during pregnancy, and the CEREBYX dosage should be adjusted as necessary. Postpartum restoration of the original dosage will probably be indicated [see Use in Specific Populations (8.1)]. Because of potential changes in protein binding during pregnancy, the monitoring of phenytoin serum levels should be based on the unbound fraction.
-
3 DOSAGE FORMS AND STRENGTHS
CEREBYX Injection is a clear, colorless to pale yellow solution available as 50 mg phenytoin sodium equivalents (PE) per mL in:
- 10 mL single-dose injection vials, each containing 500 mg phenytoin sodium equivalents
- 2 mL single-dose injection vials, each containing 100 mg phenytoin sodium equivalents
-
4 CONTRAINDICATIONS
CEREBYX is contraindicated in patients with:
- A history of hypersensitivity to CEREBYX or its inactive ingredients, or to phenytoin or other hydantoins [see Warnings and Precautions (5.6)]. Reactions have included angioedema.
- Sinus bradycardia, sino-atrial block, second and third degree A-V block, or Adams-Stokes syndrome because of the effect of parenteral phenytoin or CEREBYX on ventricular automaticity.
- A history of prior acute hepatotoxicity attributable to CEREBYX or phenytoin [see Warnings and Precautions (5.8)].
- Coadministration with delavirdine because of the potential for loss of virologic response and possible resistance to delavirdine or to the class of non-nucleoside reverse transcriptase inhibitors.
-
5 WARNINGS AND PRECAUTIONS
5.1 Dosing Errors
Phenytoin Sodium Equivalents (PE)
Do not confuse the amount of drug to be given in PE with the concentration of the drug in the vial.
Doses of CEREBYX are always expressed in terms of milligrams of phenytoin sodium equivalents (mg PE). 1 mg PE is equivalent to 1 mg phenytoin sodium.
Do not, therefore, make any adjustment in the recommended doses when substituting CEREBYX for phenytoin sodium or vice versa. For example, if a patient is receiving 1000 mg PE of CEREBYX, that is equivalent to 1000 mg of phenytoin sodium.
Concentration of 50 mg PE/mL
Medication errors associated with CEREBYX have resulted in patients receiving the wrong dose of fosphenytoin. CEREBYX is marketed in 2 mL vials containing a total of 100 mg PE and 10 mL vials containing a total of 500 mg PE. The concentration of each vial is 50 mg PE/mL. Errors have occurred when the concentration of the vial (50 mg PE/mL) was misinterpreted to mean that the total content of the vial was 50 mg PE. These errors have resulted in two- or ten-fold overdoses of CEREBYX since each vial actually contains a total of 100 mg PE or 500 mg PE. In some cases, ten-fold overdoses were associated with fatal outcomes. To help minimize confusion, the prescribed dose of CEREBYX should always be expressed in milligrams of phenytoin equivalents (mg PE) [see Dosage and Administration (2.1)]. Additionally, when ordering and storing CEREBYX, consider displaying the total drug content (i.e., 100 mg PE/ 2 mL or 500 mg PE/ 10 mL) instead of concentration in computer systems, pre-printed orders, and automated dispensing cabinet databases to help ensure that total drug content can be clearly identified. Care should be taken to ensure the appropriate volume of CEREBYX is withdrawn from the vial when preparing the drug for administration. Attention to these details may prevent some CEREBYX medication errors from occurring.
5.2 Cardiovascular Risk Associated with Rapid Infusion
Rapid intravenous administration of CEREBYX increases the risk of adverse cardiovascular reactions, including severe hypotension and cardiac arrhythmias. Cardiac arrhythmias have included bradycardia, heart block, QT interval prolongation, ventricular tachycardia, and ventricular fibrillation which have resulted in asystole, cardiac arrest, and death. Severe complications are most commonly encountered in critically ill patients, elderly patients, and patients with hypotension and severe myocardial insufficiency. However, cardiac events have also been reported in adults and children without underlying cardiac disease or comorbidities and at recommended doses and infusion rates.
The rate of intravenous CEREBYX administration should not exceed 150 mg phenytoin sodium equivalents (PE) per minute in adults and 2 mg PE/kg/min (or 150 mg PE/min, whichever is slower) in pediatric patients [see Dosage and Administration (2.3, 2.4)].
Although the risk of cardiovascular toxicity increases with infusion rates above the recommended infusion rate, these events have also been reported at or below the recommended infusion rate.
As non-emergency therapy, intravenous CEREBYX should be administered more slowly. Because of the risks of cardiac and local toxicity associated with IV CEREBYX, oral phenytoin should be used whenever possible.
Because adverse cardiovascular reactions have occurred during and after infusions, careful cardiac and respiratory monitoring is needed during and after the administration of intravenous CEREBYX. Reduction in rate of administration or discontinuation of dosing may be needed.
5.3 Withdrawal Precipitated Seizure, Status Epilepticus
Antiepileptic drugs should not be abruptly discontinued because of the possibility of increased seizure frequency, including status epilepticus. When, in the judgment of the clinician, the need for dosage reduction, discontinuation, or substitution of alternative antiepileptic medication arises, this should be done gradually. However, in the event of an allergic or hypersensitivity reaction, rapid substitution of alternative therapy may be necessary. In this case, alternative therapy should be an antiepileptic drug not belonging to the hydantoin chemical class.
5.4 Serious Dermatologic Reactions
CEREBYX can cause severe cutaneous adverse reactions (SCARs), which may be fatal. Reported reactions in phenytoin (the active metabolite of CEREBYX)-treated patients have included toxic epidermal necrolysis (TEN), Stevens-Johnson syndrome (SJS), acute generalized exanthematous pustulosis (AGEP), and Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) [see Warnings and Precautions (5.5)]. The onset of symptoms is usually within 28 days, but can occur later. CEREBYX should be discontinued at the first sign of a rash, unless the rash is clearly not drug-related. If signs or symptoms suggest a severe cutaneous adverse reaction, use of this drug should not be resumed and alternative therapy should be considered. If a rash occurs, the patient should be evaluated for signs and symptoms of SCARs.
Studies in patients of Chinese ancestry have found a strong association between the risk of developing SJS/TEN and the presence of HLA-B*1502, an inherited allelic variant of the HLA B gene, in patients using carbamazepine. Limited evidence suggests that HLA-B*1502 may be a risk factor for the development of SJS/TEN in patients of Asian ancestry taking other antiepileptic drugs associated with SJS/TEN, including phenytoin. Consideration should be given to avoiding CEREBYX as an alternative for carbamazepine patients positive for HLA-B*1502.
The use of HLA-B*1502 genotyping has important limitations and must never substitute for appropriate clinical vigilance and patient management. The role of other possible factors in the development of, and morbidity from, SJS/TEN, such as antiepileptic drug (AED) dose, compliance, concomitant medications, comorbidities, and the level of dermatologic monitoring have not been studied.
5.5 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as Multiorgan hypersensitivity, has been reported in patients taking antiepileptic drugs, including phenytoin and CEREBYX. Some of these events have been fatal or life-threatening. DRESS typically, although not exclusively, presents with fever, rash, lymphadenopathy, and/or facial swelling, in association with other organ system involvement, such as hepatitis, nephritis, hematological abnormalities, myocarditis, or myositis sometimes resembling an acute viral infection. Eosinophilia is often present. Because this disorder is variable in its expression, other organ systems not noted here may be involved. It is important to note that early manifestations of hypersensitivity, such as fever or lymphadenopathy, may be present even though rash is not evident. If such signs or symptoms are present, the patient should be evaluated immediately. CEREBYX should be discontinued if an alternative etiology for the signs or symptoms cannot be established.
5.6 Hypersensitivity
CEREBYX and other hydantoins are contraindicated in patients who have experienced phenytoin hypersensitivity [see Contraindications (4) and Warnings and Precautions (5.7)]. Additionally, consider alternatives to structurally similar drugs such as carboxamides (e.g., carbamazepine), barbiturates, succinimides, and oxazolidinediones (e.g., trimethadione) in these same patients. Similarly, if there is a history of hypersensitivity reactions to these structurally similar drugs in the patient or immediate family members, consider alternatives to CEREBYX.
5.7 Angioedema
Angioedema has been reported in patients treated with phenytoin and CEREBYX in the postmarketing setting. CEREBYX should be discontinued immediately if symptoms of angioedema, such as facial, perioral, or upper airway swelling occur. CEREBYX should be discontinued permanently if a clear alternative etiology for the reaction cannot be established.
5.8 Hepatic Injury
Cases of acute hepatotoxicity, including infrequent cases of acute hepatic failure, have been reported with phenytoin (the active metabolite of CEREBYX). These events may be part of the spectrum of DRESS or may occur in isolation [see Warnings and Precautions (5.5)]. Other common manifestations include jaundice, hepatomegaly, elevated serum transaminase levels, leukocytosis, and eosinophilia. The clinical course of acute phenytoin hepatotoxicity ranges from prompt recovery to fatal outcomes. In these patients with acute hepatotoxicity, CEREBYX should be immediately discontinued and not re-administered.
5.9 Hematopoietic Complications
Hematopoietic complications, some fatal, have occasionally been reported in association with administration of phenytoin (the active metabolite of CEREBYX). These have included thrombocytopenia, leukopenia, granulocytopenia, agranulocytosis, and pancytopenia with or without bone marrow suppression.
There have been a number of reports that have suggested a relationship between phenytoin and the development of lymphadenopathy (local or generalized), including benign lymph node hyperplasia, pseudolymphoma, lymphoma, and Hodgkin's disease. Although a cause and effect relationship has not been established, the occurrence of lymphadenopathy indicates the need to differentiate such a condition from other types of lymph node pathology. Lymph node involvement may occur with or without symptoms and signs resembling DRESS [see Warnings and Precautions (5.5)].
In all cases of lymphadenopathy, follow-up observation for an extended period is indicated and every effort should be made to achieve seizure control using alternative antiepileptic drugs.
5.10 Sensory Disturbances
Severe burning, itching, and/or paresthesia were reported by 7 of 16 normal volunteers administered IV CEREBYX at a dose of 1200 mg PE at the maximum rate of administration (150 mg PE/min). The severe sensory disturbance lasted from 3 to 50 minutes in 6 of these subjects and for 14 hours in the seventh subject. In some cases, milder sensory disturbances persisted for as long as 24 hours. The location of the discomfort varied among subjects with the groin mentioned most frequently as an area of discomfort. In a separate cohort of 16 normal volunteers (taken from 2 other studies) who were administered IV CEREBYX at a dose of 1200 mg PE at the maximum rate of administration (150 mg PE/min), none experienced severe disturbances, but most experienced mild to moderate itching or tingling. Patients administered CEREBYX at doses of 20 mg PE/kg at 150 mg PE/min are expected to experience discomfort of some degree. The occurrence and intensity of the discomfort can be lessened by slowing or temporarily stopping the infusion. The effect of continuing infusion unaltered in the presence of these sensations is unknown. No permanent sequelae have been reported thus far. The pharmacologic basis for these positive sensory phenomena is unknown, but other phosphate ester drugs, which deliver smaller phosphate loads, have been associated with burning, itching, and/or tingling predominantly in the groin area.
5.11 Local Toxicity (Including Purple Glove Syndrome)
Edema, discoloration, and pain distal to the site of injection (described as "purple glove syndrome") have also been reported following peripheral intravenous CEREBYX injection. This may or may not be associated with extravasation. The syndrome may not develop for several days after injection.
5.12 Phosphate Load
The phosphate load provided by CEREBYX (0.0037 mmol phosphate/mg PE CEREBYX) should be considered when treating patients who require phosphate restriction, such as those with severe renal impairment.
5.13 Renal or Hepatic Disease or Hypoalbuminemia
Because the fraction of unbound phenytoin (the active metabolite of CEREBYX) is increased in patients with renal or hepatic disease, or in those with hypoalbuminemia, the monitoring of phenytoin serum levels should be based on the unbound fraction in those patients. After IV administration to patients with renal and/or hepatic disease, or in those with hypoalbuminemia, fosphenytoin clearance to phenytoin may be increased without a similar increase in phenytoin clearance. This has the potential to increase the frequency and severity of adverse events.
5.14 Exacerbation of Porphyria
In view of isolated reports associating phenytoin (the active metabolite of CEREBYX) with exacerbation of porphyria, caution should be exercised in using CEREBYX in patients suffering from this disease.
5.15 Teratogenicity and Other Harm to the Newborn
CEREBYX may cause fetal harm when administered to a pregnant woman. Prenatal exposure to phenytoin (the active metabolite of CEREBYX) may increase the risks for congenital malformations and other adverse developmental outcomes [see Use in Specific Populations (8.1)].
Increased frequencies of major malformations (such as orofacial clefts and cardiac defects), and abnormalities characteristic of fetal hydantoin syndrome, including dysmorphic skull and facial features, nail and digit hypoplasia, growth abnormalities (including microcephaly), and cognitive deficits, have been reported among children born to epileptic women who took phenytoin alone or in combination with other antiepileptic drugs during pregnancy. There have been several reported cases of malignancies, including neuroblastoma.
A potentially life-threatening bleeding disorder related to decreased levels of vitamin K-dependent clotting factors may occur in newborns exposed to phenytoin in utero. This drug-induced condition can be prevented with vitamin K administration to the mother before delivery and to the neonate after birth.
5.16 Slow Metabolizers of Phenytoin
A small percentage of individuals who have been treated with phenytoin (the active metabolite of CEREBYX) have been shown to metabolize the drug slowly. Slow metabolism may be caused by limited enzyme availability and lack of induction; it appears to be genetically determined. If early signs of dose-related central nervous system (CNS) toxicity develop, serum levels should be checked immediately.
5.17 Hyperglycemia
Hyperglycemia, resulting from the inhibitory effect of phenytoin (the active metabolite of CEREBYX) on insulin release, has been reported. Phenytoin may also raise the serum glucose concentrations in diabetic patients.
5.18 Serum Phenytoin Levels above Therapeutic Range
Serum levels of phenytoin (the active metabolite of CEREBYX) sustained above the therapeutic range may produce confusional states referred to as "delirium," "psychosis," or "encephalopathy," or rarely, irreversible cerebellar dysfunction and/or cerebellar atrophy. Accordingly, at the first sign of acute toxicity, serum levels should be immediately checked. CEREBYX dose reduction is indicated if serum levels are excessive; if symptoms persist, administration of CEREBYX should be discontinued.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Cardiovascular Risk Associated with Rapid Infusion [see Warnings and Precautions (5.2)]
- Withdrawal Precipitated Seizure, Status Epilepticus [see Warnings and Precautions (5.3)]
- Serious Dermatologic Reactions [see Warnings and Precautions (5.4)]
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity [see Warnings and Precautions (5.5)]
- Hypersensitivity [see Warnings and Precautions (5.6)]
- Angioedema [see Warnings and Precautions (5.7)]
- Hepatic Injury [see Warnings and Precautions (5.8)]
- Hematopoietic Complications [see Warnings and Precautions (5.9)]
- Sensory Disturbances [see Warnings and Precautions (5.10)]
- Local Toxicity (Including Purple Glove Syndrome) [see Warnings and Precautions (5.11)]
- Exacerbation of Porphyria [see Warnings and Precautions (5.14)]
- Teratogenicity and Other Harm to the Newborn [see Warnings and Precautions (5.15)]
- Hyperglycemia [see Warnings and Precautions (5.17)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The more important adverse clinical reactions caused by the IV use of CEREBYX or phenytoin are cardiovascular collapse and/or CNS depression. Hypotension can occur when either drug is administered rapidly by the IV route. The rate of administration is very important; for CEREBYX, it should not exceed 150 mg PE/min [see Warnings and Precautions (5.2)]. The adverse reactions most commonly observed with the use of CEREBYX in clinical trials were nystagmus, dizziness, pruritus, somnolence, and ataxia. With one exception, these reactions are commonly associated with the administration of IV phenytoin. Pruritus, however, was seen much more often following CEREBYX administration and occurred more often with IV CEREBYX administration than with IM CEREBYX administration. These reactions were dose and rate related; most alert patients (41 of 64; 64%) administered doses of ≥15 mg PE/kg at 150 mg PE/min experienced discomfort of some degree. These sensations, generally described as itching, burning, or tingling, were usually not at the infusion site. The location of the discomfort varied with the groin mentioned most frequently as a site of involvement. The paresthesia and pruritus were transient events that occurred within several minutes of the start of infusion and generally resolved within 10 minutes after completion of CEREBYX infusion. Some patients experienced symptoms for hours. These reactions did not increase in severity with repeated administration. Concurrent adverse events or clinical laboratory change suggesting an allergic process were not seen [see Warnings and Precautions (5.10)]. Approximately 2% of the 859 patients who received CEREBYX in premarketing clinical trials discontinued treatment because of an adverse event. The adverse events most commonly associated with withdrawal were pruritus (0.5%), hypotension (0.3%), and bradycardia (0.2%).
Dose and Rate Dependency of Adverse Reactions Following IV CEREBYX: The incidence of adverse reactions tended to increase as both dose and infusion rate increased. In particular, at doses of ≥15mg PE/kg and rates ≥150 mg PE/min, transient pruritus, tinnitus, nystagmus, somnolence, and ataxia occurred 2 to 3 times more often than at lower doses or rates.
Incidence in Controlled Clinical Trials
All adverse events were recorded during the trials by the clinical investigators using terminology of their own choosing. Similar types of events were grouped into standardized categories using modified COSTART dictionary terminology. These categories are used in the tables and listings below with the frequencies representing the proportion of individuals exposed to CEREBYX or comparative therapy.
Incidence in Controlled Clinical Trials - IV Administration to Adult Patients with Epilepsy or Neurosurgical Patients: Table 4 lists adverse reactions that occurred in at least 2% of patients treated with IV CEREBYX at the maximum dose and rate in a randomized, double-blind, controlled clinical trial where the rates for phenytoin and CEREBYX administration would have resulted in equivalent systemic exposure to phenytoin.
TABLE 4. Adverse Reaction Incidence Following IV Administration at the Maximum Dose and Rate to Adult Patients with Epilepsy or Neurosurgical Patients (Events in at Least 2% of CEREBYX-Treated Patients) BODY SYSTEM IV CEREBYX IV Phenytoin* Adverse Event N=90 N=22 - * The study was not designed to assess comparative safety.
BODY AS A WHOLE Pelvic Pain 4 0 Asthenia 2 0 Back Pain 2 0 Headache 2 5 CARDIOVASCULAR Hypotension 8 9 Vasodilatation 6 5 Tachycardia 2 0 DIGESTIVE Nausea 9 14 Tongue Disorder 4 0 Dry Mouth 4 5 Vomiting 2 9 NERVOUS Nystagmus 44 59 Dizziness 31 27 Somnolence 20 27 Ataxia 11 18 Stupor 8 5 Incoordination 4 5 Paresthesia 4 0 Extrapyramidal Syndrome 4 0 Tremor 3 9 Agitation 3 0 Hypesthesia 2 9 Dysarthria 2 0 Vertigo 2 0 Brain Edema 2 5 SKIN AND APPENDAGES Pruritus 49 5 SPECIAL SENSES Tinnitus 9 9 Diplopia 3 0 Taste Perversion 3 0 Amblyopia 2 9 Deafness 2 0 Incidence in Clinical Trials - IV Administration to Pediatric Patients with Epilepsy or Neurosurgical Patients: The overall incidence of adverse reactions and the types of adverse reactions seen were similar among children and adults treated with CEREBYX. In an open-label, safety, tolerability, and pharmacokinetic study of fosphenytoin in pediatric subjects (neonates through age 16), the following adverse reactions occurred at a frequency of at least 5% in 96 subjects treated with intravenous CEREBYX: vomiting (21%), nystagmus (18%), ataxia (10%), fever (8%), nervousness (7%), pruritus (6%), somnolence (6%), hypotension (5%), and rash (5%).
Incidence in Controlled Trials - IM Administration to Adult Patients with Epilepsy: Table 5 lists adverse reactions that occurred in at least 2% of CEREBYX-treated patients in a double-blind, randomized, controlled clinical trial of adult epilepsy patients receiving either IM CEREBYX substituted for oral phenytoin or continuing oral phenytoin. Both treatments were administered for 5 days.
TABLE 5. Adverse Reaction Incidence Following Substitution of IM CEREBYX for Oral Phenytoin in Adult Patients with Epilepsy (Events in at Least 2% of CEREBYX-Treated Patients) BODY SYSTEM IM CEREBYX Oral Phenytoin* Adverse Event N=179 N=61 - * The study was not designed to assess comparative safety.
BODY AS A WHOLE Headache 9 5 Asthenia 9 3 DIGESTIVE Nausea 5 0 Vomiting 3 0 HEMATOLOGIC AND LYMPHATIC Ecchymosis 7 5 NERVOUS Nystagmus 15 8 Tremor 10 13 Ataxia 8 8 Incoordination 8 5 Somnolence 7 10 Dizziness 5 3 Paresthesia 4 3 Reflexes Decreased 3 5 SKIN AND APPENDAGES Pruritus 3 0 Adverse Events During Clinical Trials in Adult and Pediatric Patients
CEREBYX has been administered to approximately 900 individuals during clinical trials. Adverse events seen at least twice are listed in the following, except those already included in previous tables and listings. Events are further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: frequent adverse events are defined as those occurring in greater than 1/100 individuals; infrequent adverse events are those occurring in 1/100 to 1/1000 individuals.
Body as a Whole: Frequent: fever, injection-site reaction, infection, chills, face edema, injection-site pain; Infrequent: sepsis, injection-site inflammation, injection-site edema, injection-site hemorrhage, flu syndrome, malaise, generalized edema, shock, photosensitivity reaction, cachexia, cryptococcosis.
Cardiovascular: Frequent: hypertension; Infrequent: cardiac arrest, migraine, syncope, cerebral hemorrhage, palpitation, sinus bradycardia, atrial flutter, bundle branch block, cardiomegaly, cerebral infarct, postural hypotension, pulmonary embolus, QT interval prolongation, thrombophlebitis, ventricular extrasystoles, congestive heart failure.
Digestive: Frequent: constipation; Infrequent: dyspepsia, diarrhea, anorexia, gastrointestinal hemorrhage, increased salivation, liver function tests abnormal, tenesmus, tongue edema, dysphagia, flatulence, gastritis, ileus.
Endocrine: Infrequent: diabetes insipidus.
Hematologic and Lymphatic: Infrequent: thrombocytopenia, anemia, leukocytosis, cyanosis, hypochromic anemia, leukopenia, lymphadenopathy, petechia.
Laboratory Test Abnormality: Phenytoin (the active metabolite of CEREBYX) may cause increased serum levels of glucose and alkaline phosphatase.
Metabolic and Nutritional: Frequent: hypokalemia; Infrequent: hyperglycemia, hypophosphatemia, alkalosis, acidosis, dehydration, hyperkalemia, ketosis.
Musculoskeletal: Frequent: myasthenia; Infrequent: myopathy, leg cramps, arthralgia, myalgia.
Nervous: Frequent: reflexes increased, speech disorder, dysarthria, intracranial hypertension, thinking abnormal, nervousness; Infrequent: confusion, twitching, Babinski sign positive, circumoral paresthesia, hemiplegia, hypotonia, convulsion, extrapyramidal syndrome, insomnia, meningitis, depersonalization, CNS depression, depression, hypokinesia, hyperkinesia, paralysis, psychosis, aphasia, emotional lability, coma, hyperesthesia, myoclonus, personality disorder, acute brain syndrome, encephalitis, subdural hematoma, encephalopathy, hostility, akathisia, amnesia, neurosis.
Respiratory: Frequent: pneumonia; Infrequent: pharyngitis, sinusitis, hyperventilation, rhinitis, apnea, aspiration pneumonia, asthma, dyspnea, atelectasis, cough increased, sputum increased, epistaxis, hypoxia, pneumothorax, hemoptysis, bronchitis.
Skin and Appendages: Frequent: rash; Infrequent: maculopapular rash, urticaria, sweating, skin discoloration, contact dermatitis, pustular rash, skin nodule.
Special Senses: Infrequent: visual field defect, eye pain, conjunctivitis, photophobia, hyperacusis, mydriasis, parosmia, ear pain, taste loss.
Urogenital: Infrequent: urinary retention, oliguria, dysuria, vaginitis, albuminuria, genital edema, kidney failure, polyuria, urethral pain, urinary incontinence, vaginal moniliasis.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of fosphenytoin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a Whole: Anaphylaxis, angioedema [see Warnings and Precautions (5.7)]
Laboratory Test Abnormality: Phenytoin or CEREBYX may decrease serum concentrations of T4. It may also produce lower than normal values for dexamethasone or metyrapone tests. Phenytoin may also cause increased serum levels of gamma glutamyl transpeptidase (GGT).
Nervous System Disorders: Dyskinesia
-
7 DRUG INTERACTIONS
Fosphenytoin is extensively bound to human plasma proteins. Drugs highly bound to albumin could increase the unbound fraction of fosphenytoin. Although, it is unknown whether this could result in clinically significant effects, caution is advised when administering CEREBYX with other drugs that significantly bind to serum albumin. The most significant drug interactions following administration of CEREBYX are expected to occur with drugs that interact with phenytoin. Phenytoin is extensively bound to serum plasma proteins and is prone to competitive displacement. Phenytoin is metabolized by hepatic cytochrome P450 enzymes CYP2C9 and CYP2C19 and is particularly susceptible to inhibitory drug interactions because it is subject to saturable metabolism. Inhibition of metabolism may produce significant increases in circulating phenytoin concentrations and enhance the risk of drug toxicity. Monitoring of phenytoin serum levels is recommended when a drug interaction is suspected.
Phenytoin or CEREBYX is a potent inducer of hepatic drug-metabolizing enzymes.
7.1 Drugs that Affect Phenytoin or CEREBYX
Table 6 includes commonly occurring drug interactions that affect phenytoin (the active metabolite of CEREBYX) concentrations. However, this list is not intended to be inclusive or comprehensive. Individual prescribing information from relevant drugs should be consulted.
The addition or withdrawal of these agents in patients on phenytoin therapy may require an adjustment of the phenytoin dose to achieve optimal clinical outcome.
Table 6. Drugs That Affect Phenytoin Concentrations Interacting Agent Examples - * The induction potency of St. John's wort may vary widely based on preparation.
Drugs that may increase phenytoin serum levels Antiepileptic drugs Ethosuximide, felbamate, oxcarbazepine, methsuximide, topiramate Azoles Fluconazole, ketoconazole, itraconazole, miconazole, voriconazole Antineoplastic agents Capecitabine, fluorouracil Antidepressants Fluoxetine, fluvoxamine, sertraline Gastric acid reducing agents H2 antagonists (cimetidine), omeprazole Sulfonamides Sulfamethizole, sulfaphenazole, sulfadiazine, sulfamethoxazole-trimethoprim Other Acute alcohol intake, amiodarone, chloramphenicol, chlordiazepoxide, disulfiram, estrogen, fluvastatin, isoniazid, methylphenidate, phenothiazines, salicylates, ticlopidine, tolbutamide, trazodone, warfarin Drugs that may decrease phenytoin serum levels Antineoplastic agents usually in combination Bleomycin, carboplatin, cisplatin, doxorubicin, methotrexate Antiviral agents Fosamprenavir, nelfinavir, ritonavir Antiepileptic drugs Carbamazepine, vigabatrin Other Chronic alcohol abuse, diazepam, diazoxide, folic acid, reserpine, rifampin, St. John's wort,* theophylline Drugs that may either increase or decrease phenytoin serum levels Antiepileptic drugs Phenobarbital, valproate sodium, valproic acid 7.2 Drugs Affected by Phenytoin or CEREBYX
Table 7 includes commonly occurring drug interactions affected by phenytoin (the active metabolite of CEREBYX). However, this list is not intended to be inclusive or comprehensive. Individual drug package inserts should be consulted. The addition or withdrawal of phenytoin during concomitant therapy with these agents may require adjustment of the dose of these agents to achieve optimal clinical outcome.
Table 7: Drugs Affected by Phenytoin Interacting Agent Examples - * The effect of phenytoin on phenobarbital, valproic acid and sodium valproate serum levels is unpredictable.
Drugs whose efficacy is impaired by phenytoin Azoles Fluconazole, ketoconazole, itraconazole, posaconazole, voriconazole Antineoplastic agents Irinotecan, paclitaxel, teniposide Delavirdine Phenytoin can substantially reduce the concentrations of delavirdine. This can lead to loss of virologic response and possible resistance [see Contraindications (4)]. Neuromuscular blocking agents Cisatracurium, pancuronium, rocuronium and vecuronium: resistance to the neuromuscular blocking action of the nondepolarizing neuromuscular blocking agents has occurred in patients chronically administered phenytoin. Whether or not phenytoin has the same effect on other non-depolarizing agents is unknown. - Prevention or Management: Patients should be monitored closely for more rapid recovery from neuromuscular blockade than expected, and infusion rate requirements may be higher.
Warfarin Increased and decreased PT/INR responses have been reported when phenytoin is coadministered with warfarin. Other Corticosteroids, doxycycline, estrogens, furosemide, oral contraceptives, paroxetine, quinidine, rifampin, sertraline, theophylline, and vitamin D Drugs whose level is decreased by phenytoin Antiepileptic drugs* Carbamazepine, felbamate, lamotrigine, topiramate, oxcarbazepine Antilipidemic agents Atorvastatin, fluvastatin, simvastatin Antiviral agents Efavirenz, lopinavir/ritonavir, indinavir, nelfinavir, ritonavir, saquinavir
Fosamprenavir: phenytoin when given with fosamprenavir alone may decrease the concentration of amprenavir, the active metabolite. Phenytoin when given with the combination of fosamprenavir and ritonavir may increase the concentration of amprenavirCalcium channel blockers Nifedipine, nimodipine, nisoldipine, verapamil Other Albendazole (decreases active metabolite), chlorpropamide, clozapine, cyclosporine, digoxin, disopyramide, folic acid, methadone, mexiletine, praziquantel, quetiapine -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antiepileptic drugs (AEDs), such as CEREBYX, during pregnancy. Physicians are advised to recommend that pregnant patients taking CEREBYX enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry. This can be done by calling the toll free number 1-888-233-2334, and must be done by patients themselves. Information on the registry can also be found at the website http://www.aedpregnancyregistry.org/.
Risk Summary
In humans, prenatal exposure to phenytoin (the active metabolite of CEREBYX) may increase the risks for congenital malformations and other adverse developmental outcomes. Prenatal phenytoin exposure is associated with an increased incidence of major malformations, including orofacial clefts and cardiac defects. In addition, the fetal hydantoin syndrome, a pattern of abnormalities including dysmorphic skull and facial features, nail and digit hypoplasia, growth abnormalities (including microcephaly), and cognitive deficits has been reported among children born to epileptic women who took phenytoin alone or in combination with other antiepileptic drugs during pregnancy [see Data]. There have been several reported cases of malignancies, including neuroblastoma, in children whose mothers received phenytoin during pregnancy.
Administration of phenytoin to pregnant animals resulted in an increased incidence of fetal malformations and other manifestations of developmental toxicity (including embryofetal death, growth impairment, and behavioral abnormalities) in multiple species at clinically relevant doses [see Data].
In the U.S. general population, the estimated background risk of major birth defects and of miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Clinical Considerations
Disease-associated maternal risk
An increase in seizure frequency may occur during pregnancy because of altered phenytoin pharmacokinetics. Periodic measurement of serum phenytoin concentrations may be valuable in the management of pregnant women as a guide to appropriate adjustment of dosage [see Dosage and Administration (2.5, 2.9)]. However, postpartum restoration of the original dosage will probably be indicated.
Fetal/Neonatal adverse reactions
A potentially life-threatening bleeding disorder related to decreased levels of vitamin K-dependent clotting factors may occur in newborns exposed to phenytoin in utero. This drug-induced condition can be prevented with vitamin K administration to the mother before delivery and to the neonate after birth.
Data
Human Data
Meta-analyses using data from published observational studies and registries have estimated an approximately 2.4-fold increased risk for any major malformation in children with prenatal phenytoin exposure compared to controls. An increased risk of heart defects, facial clefts, and digital hypoplasia has been reported. The fetal hydantoin syndrome is a pattern of congenital anomalies including craniofacial anomalies, nail and digital hypoplasia, prenatal-onset growth deficiency, and neurodevelopmental deficiencies.
Animal Data
Administration of phenytoin to pregnant rats, rabbits, and mice during organogenesis resulted in embryofetal death, fetal malformations, and decreased fetal growth. Malformations (including craniofacial, cardiovascular, neural, limb, and digit abnormalities) were observed in rats, rabbits, and mice at doses as low as 100, 75, and 12.5 mg/kg, respectively.
8.2 Lactation
Risk Summary
It is not known whether fosphenytoin is secreted in human milk. Following administration of phenytoin (the active metabolite of CEREBYX), phenytoin is secreted in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for CEREBYX and any potential adverse effects on the breastfed infant from CEREBYX or from the underlying maternal condition.
8.4 Pediatric Use
CEREBYX is indicated for the treatment of generalized tonic-clonic status epilepticus and prevention and treatment of seizures occurring during neurosurgery in all pediatric age groups [see Indications and Usage (1) and Dosage and Administration (2.3, 2.4)]. Because rapid intravenous administration of CEREBYX increases the risk of adverse cardiovascular reactions, the rate of administration should not exceed 2 mg PE/kg/min (or 150 mg PE/min, whichever is slower) in pediatric patients [see Dosage and Administration (2.3, 2.4) and Warnings and Precautions (5.2)].
8.5 Geriatric Use
No systematic studies in geriatric patients have been conducted. Phenytoin clearance tends to decrease with increasing age [see Clinical Pharmacology (12.3)]. Lower or less frequent dosing may be required [see Clinical Pharmacology (12.3) and Dosage and Administration (2.8)].
8.6 Renal and/or Hepatic Impairment, or Hypoalbuminemia
The liver is the site of biotransformation. Patients with impaired liver function, elderly patients, or those who are gravely ill may show early toxicity.
Because the fraction of unbound phenytoin (the active metabolite of CEREBYX) is increased in patients with renal or hepatic disease, or in those with hypoalbuminemia, the monitoring of phenytoin serum levels should be based on the unbound fraction in those patients.
After IV administration to patients with renal and/or hepatic disease, or in those with hypoalbuminemia, fosphenytoin clearance to phenytoin may be increased without a similar increase in phenytoin clearance. This has the potential to increase the frequency and severity of adverse events.
-
10 OVERDOSAGE
Nausea, vomiting, lethargy, tachycardia, bradycardia, asystole, cardiac arrest, hypotension, syncope, hypocalcemia, metabolic acidosis, and death have been reported in cases of overdosage with CEREBYX.
Because CEREBYX is a prodrug of phenytoin, the following information about phenytoin overdosage may be helpful. Initial symptoms of acute phenytoin toxicity are nystagmus, ataxia, and dysarthria. Other signs include tremor, hyperreflexia, lethargy, slurred speech, nausea, vomiting, coma, and hypotension. Death is caused by respiratory and circulatory depression. The lethal dose of phenytoin in adults is estimated to be 2 to 5 grams. The lethal dose in pediatrics is not known.
There are marked variations among individuals with respect to serum phenytoin concentrations where toxicity occurs. Lateral gaze nystagmus usually appears at 20 µg/mL, ataxia at 30 µg/mL, and dysarthria and lethargy appear when the serum concentration is over 40 µg/mL. However, phenytoin concentrations as high as 50 µg/mL have been reported without evidence of toxicity. As much as 25 times the therapeutic phenytoin dose has been taken, resulting in serum phenytoin concentrations over 100 µg/mL, with complete recovery. Irreversible cerebellar dysfunction and atrophy have been reported after overdosage.
Formate and phosphate are metabolites of CEREBYX and therefore may contribute to signs of toxicity following overdosage. Signs of formate toxicity are similar to those of methanol toxicity and are associated with severe anion-gap metabolic acidosis. Large amounts of phosphate, delivered rapidly, could potentially cause hypocalcemia with paresthesia, muscle spasms, and seizures. Ionized free calcium levels can be measured and, if low, used to guide treatment.
Treatment: Treatment is nonspecific since there is no known antidote to CEREBYX or phenytoin overdosage.
The adequacy of the respiratory and circulatory systems should be carefully observed, and appropriate supportive measures employed. Hemodialysis can be considered since phenytoin (the active metabolite of CEREBYX) is not completely bound to plasma proteins. Total exchange transfusion has been used in the treatment of severe intoxication in children.
In acute overdosage the possibility of other CNS depressants, including alcohol, should be borne in mind.
-
11 DESCRIPTION
CEREBYX® (fosphenytoin sodium injection) is a prodrug intended for parenteral administration; its active metabolite is phenytoin. 1.5 mg of fosphenytoin sodium is equivalent to 1 mg phenytoin sodium, and is referred to as 1 mg phenytoin sodium equivalents (PE). The amount and concentration of fosphenytoin is always expressed in terms of mg PE.
The pharmacological class of the fosphenytoin sodium is hydantoin derivative, and the therapeutic class is anticonvulsant.
CEREBYX is marketed in 2 mL vials containing a total of 100 mg PE and 10 mL vials containing a total of 500 mg PE, for intravenous or intramuscular administration. The concentration of each vial is 50 mg PE/mL. CEREBYX is supplied in vials as a sterile solution in Water for Injection, USP, and Tromethamine, USP (TRIS), buffer adjusted to pH 8.6 to 9.0 with either Hydrochloric Acid, NF, or Sodium Hydroxide, NF. CEREBYX is a clear, colorless to pale yellow, sterile solution.
The chemical name of fosphenytoin is 5,5-diphenyl-3-[(phosphonooxy)methyl]-2,4-imidazolidinedione disodium salt. The molecular structure of fosphenytoin is:
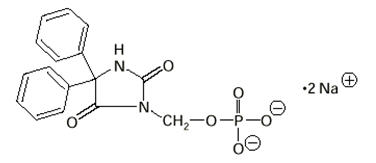
The molecular weight of fosphenytoin is 406.24.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Fosphenytoin is a prodrug of phenytoin and accordingly, its anticonvulsant effects are attributable to phenytoin. The precise mechanism by which phenytoin exerts its therapeutic effect has not been established but is thought to involve the voltage-dependent blockade of membrane sodium channels resulting in a reduction in sustained high-frequency neuronal discharges.
12.3 Pharmacokinetics
Fosphenytoin
Absorption
Intravenous: When CEREBYX is administered by IV infusion, maximum plasma fosphenytoin concentrations are achieved at the end of the infusion.
Intramuscular: Fosphenytoin is completely bioavailable following IM administration of CEREBYX. Peak concentrations occur at approximately 30 minutes postdose. Plasma fosphenytoin concentrations following IM administration are lower but more sustained than those following IV administration due to the time required for absorption of fosphenytoin from the injection site.
Distribution
Fosphenytoin is extensively bound (95% to 99%) to human plasma proteins, primarily albumin. Binding to plasma proteins is saturable with the result that the percent bound decreases as total fosphenytoin concentrations increase. Fosphenytoin displaces phenytoin from protein binding sites. The volume of distribution of fosphenytoin increases with CEREBYX dose and rate, and ranges from 4.3 to 10.8 liters.
Elimination
The conversion half-life of fosphenytoin to phenytoin is approximately 15 minutes.
Metabolism
Following parenteral administration of CEREBYX, fosphenytoin is converted to the anticonvulsant phenytoin. The mechanism of fosphenytoin conversion has not been determined, but phosphatases probably play a major role. Fosphenytoin is metabolized to phenytoin, phosphate, and formate. For every mmol of fosphenytoin administered, one mmol of phenytoin is produced. The hydrolysis of fosphenytoin to phenytoin yields two metabolites, phosphate and formaldehyde. Formaldehyde is subsequently converted to formate, which is in turn metabolized via a folate dependent mechanism. Although phosphate and formaldehyde (formate) have potentially important biological effects, these effects typically occur at concentrations considerably in excess of those obtained when CEREBYX is administered under conditions of use recommended in this labeling.
Phenytoin (after CEREBYX administration)
In general, IM administration of CEREBYX generates systemic phenytoin concentrations that are similar enough to oral phenytoin sodium to allow essentially interchangeable use. The pharmacokinetics of fosphenytoin following IV administration of CEREBYX, however, are complex, and when used in an emergency setting (e.g., status epilepticus), differences in rate of availability of phenytoin could be critical. Studies have therefore empirically determined an infusion rate for CEREBYX that gives a rate and extent of phenytoin systemic availability similar to that of a 50 mg/min phenytoin sodium infusion. A dose of 15 to 20 mg PE/kg of CEREBYX infused at 100 to 150 mg PE/min yields plasma free phenytoin concentrations over time that approximate those achieved when an equivalent dose of phenytoin sodium (e.g., parenteral DILANTIN®) is administered at 50 mg/min [see Dosage and Administration (2.3) and Warnings and Precautions (5.2)].
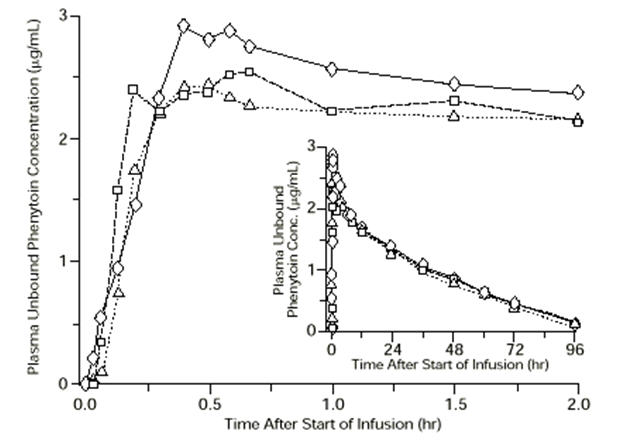
FIGURE 1. Mean plasma unbound phenytoin concentrations following IV administration of 1200 mg PE CEREBYX infused at 100 mg PE/min (triangles) or 150 mg PE/min (squares) and 1200 mg Dilantin infused at 50 mg/min (diamonds) to healthy subjects (N = 12). Inset shows time course for the entire 96-hour sampling period. Following administration of single IV CEREBYX doses of 400 to 1200 mg PE, mean maximum total phenytoin concentrations increase in proportion to dose, but do not change appreciably with changes in infusion rate. In contrast, mean maximum unbound phenytoin concentrations increase with both dose and rate.
Absorption:
Fosphenytoin is completely converted to phenytoin following IV administration, with a half-life of approximately 15 minutes. Fosphenytoin is also completely converted to phenytoin following IM administration and plasma total phenytoin concentrations peak in approximately 3 hours.
Distribution:
Phenytoin is highly bound to plasma proteins, primarily albumin, although to a lesser extent than fosphenytoin. In the absence of fosphenytoin, approximately 12% of total plasma phenytoin is unbound over the clinically relevant concentration range. However, fosphenytoin displaces phenytoin from plasma protein binding sites. This increases the fraction of phenytoin unbound (up to 30% unbound) during the period required for conversion of fosphenytoin to phenytoin (approximately 0.5 to 1 hour postinfusion).
Elimination:
Mean total phenytoin half-life values (12.0 to 28.9 hr) following CEREBYX administration at these doses are similar to those after equal doses of parenteral Dilantin and tend to be greater at higher plasma phenytoin concentrations.
Metabolism
Phenytoin derived from administration of CEREBYX is extensively metabolized in the liver by the cytochrome P450 enzymes CYP2C9 and CYP2C19. Phenytoin hepatic metabolism is saturable, and following administration of single IV CEREBYX doses of 400 to 1200 mg PE, total and unbound phenytoin AUC values increase disproportionately with dose.
Specific Populations
Age: Geriatric Population:
The effect of age on the pharmacokinetics of fosphenytoin was evaluated in patients 5 to 98 years of age. Patient age had no significant impact on fosphenytoin pharmacokinetics. Phenytoin clearance tends to decrease with increasing age (20% less in patients over 70 years of age relative to that in patients 20 to 30 years of age).
Renal or Hepatic Impairment:
Increased fraction of unbound phenytoin (the active metabolite of CEREBYX) in patients with renal or hepatic disease, or in those with hypoalbuminemia has been reported.
Pregnancy:
It has been reported in the literature that the plasma clearance of phenytoin (the active metabolite of CEREBYX) generally increased during pregnancy, reached a peak in the third trimester and returned to the level of pre-pregnancy after few weeks or months of delivery [see Dosage and Administration (2.9)].
Drug Interaction Studies
Phenytoin derived from administration of CEREBYX is extensively metabolized in the liver by the cytochrome P450 enzymes CYP2C9 and CYP2C19 [see Drug Interactions (7.1, 7.2)]. No drugs are known to interfere with the conversion of fosphenytoin to phenytoin. Conversion could be affected by alterations in the level of phosphatase activity, but given the abundance and wide distribution of phosphatases in the body it is unlikely that drugs would affect this activity enough to affect conversion of fosphenytoin to phenytoin.
The pharmacokinetics and protein binding of fosphenytoin, phenytoin, and diazepam were not altered when diazepam and CEREBYX were concurrently administered in single submaximal doses.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis [see Warnings and Precautions (5.9)]
The carcinogenic potential of fosphenytoin has not been assessed. In carcinogenicity studies, phenytoin (active metabolite of fosphenytoin) was administered in the diet to mice (10, 25, or 45 mg/kg/day) and rats (25, 50, or 100 mg/kg/day) for 2 years. The incidences of hepatocellular tumors were increased in male and female mice at the highest dose. No increases in tumor incidence were observed in rats. The highest doses tested in these studies were associated with peak plasma phenytoin levels below human therapeutic concentrations.
In carcinogenicity studies reported in the literature, phenytoin was administered in the diet for 2 years at doses up to 600 ppm (approximately 160 mg/kg/day) to mice and up to 2400 ppm (approximately 120 mg/kg/day) to rats. The incidences of hepatocellular tumors were increased in female mice at all but the lowest dose tested. No increases in tumor incidence were observed in rats.
Mutagenesis
An increase in structural chromosome aberrations were observed in cultured V79 Chinese hamster lung cells exposed to fosphenytoin in the presence of metabolic activation. No evidence of mutagenicity was observed in bacteria (Ames test) or Chinese hamster lung cells in vitro, and no evidence for clastogenic activity was observed in an in vivo mouse bone marrow micronucleus assay.
Impairment of Fertility
Fosphenytoin was administered to male and female rats during mating and continuing in females throughout gestation and lactation at doses of 50 mg PE/kg or higher. No effects on fertility were observed in males. In females, altered estrous cycles, delayed mating, prolonged gestation length, and developmental toxicity were observed at all doses, which were associated with maternal toxicity. The lowest dose tested is approximately 40% of the maximum human loading dose on a mg/m2 basis.
-
14 CLINICAL STUDIES
Infusion tolerance was evaluated in clinical studies. One double-blind study assessed infusion-site tolerance of equivalent loading doses (15 to 20 mg PE/kg) of CEREBYX infused at 150 mg PE/min or phenytoin infused at 50 mg/min. The study demonstrated better local tolerance (pain and burning at the infusion site), fewer disruptions of the infusion, and a shorter infusion period for CEREBYX-treated patients (Table 8).
TABLE 8. Infusion Tolerance of Equivalent Loading Doses of IV CEREBYX and IV Phenytoin IV CEREBYX
N=90IV Phenytoin
N=22- * Percent of patients
Local Intolerance 9%* 90% Infusion Disrupted 21% 67% Average Infusion Time 13 min 44 min CEREBYX-treated patients, however, experienced more systemic sensory disturbances [see Warnings and Precautions (5.10)]. Infusion disruptions in CEREBYX-treated patients were primarily due to systemic burning, pruritus, and/or paresthesia while those in phenytoin-treated patients were primarily due to pain and burning at the infusion site (see Table 8). In a double-blind study investigating temporary substitution of CEREBYX for oral phenytoin, IM CEREBYX was as well-tolerated as IM placebo. IM CEREBYX resulted in a slight increase in transient, mild to moderate local itching (23% of CEREBYX-treated patients vs 11% of IM placebo-treated patients at any time during the study). This study also demonstrated that equimolar doses of IM CEREBYX may be substituted for oral phenytoin sodium with no dosage adjustments needed when initiating IM or returning to oral therapy. In contrast, switching between IM and oral phenytoin requires dosage adjustments because of slow and erratic phenytoin absorption from muscle.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
CEREBYX Injection is a clear, colorless to pale yellow solution supplied as follows:
mg phenytoin sodium equivalents (PE) per vial Volume per vial (mL) Package Configuration NDC 500 mg PE/10 mL vial
10 mL per vial
Package contains 10 vials of NDC: 0069-6001-10 NDC: 0069-6001-21 100 mg PE/2 mL vial
2 mL per vial Package contains 25 vials of NDC: 0069-6001-02 NDC: 0069-6001-25 Both sizes of vials contain Tromethamine, USP (TRIS), Hydrochloric Acid, NF, or Sodium Hydroxide, NF, and Water for Injection, USP.
CEREBYX should always be prescribed in phenytoin sodium equivalents (PE) [see Dosage and Administration (2.1) and Warnings and Precautions (5.1)].
1.5 mg of fosphenytoin sodium is equivalent to 1 mg phenytoin sodium, and is referred to as 1 mg PE. The amount and concentration of fosphenytoin is always expressed in terms of mg of phenytoin sodium equivalents (PE). Fosphenytoin's weight is expressed as phenytoin sodium equivalents to avoid the need to perform molecular weight-based adjustments when substituting fosphenytoin for phenytoin or vice versa.
16.2 Storage and Handling
Store under refrigeration at 2°C to 8°C (36°F to 46°F). The product should not be stored at room temperature for more than 48 hours. Vials that develop particulate matter should not be used.
Injection vials are single-dose only. After opening, any unused product should be discarded.
-
17 PATIENT COUNSELING INFORMATION
Cardiovascular Risk Associated with Rapid Infusion
Inform patients that rapid intravenous administration of CEREBYX increases the risk of adverse cardiovascular reactions, including severe hypotension and cardiac arrhythmias. Cardiac arrhythmias have included bradycardia, heart block, ventricular tachycardia, and ventricular fibrillation which have resulted in asystole, cardiac arrest, and death. Patients should report cardiac signs or symptoms to their healthcare provider [see Warnings and Precautions (5.2)].
Withdrawal of Antiepileptic Drugs
Advise patients not to discontinue use of CEREBYX without consulting with their healthcare provider. CEREBYX should normally be gradually withdrawn to reduce the potential for increased seizure frequency and status epilepticus [see Warnings and Precautions (5.3)].
Serious Dermatologic Reactions
Advise patients of the early signs and symptoms of severe cutaneous adverse reactions and to report any occurrence immediately to a physician [see Warnings and Precautions (5.4)].
Potential Signs of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) and Other Systemic Reactions
Advise patients of the early toxic signs and symptoms of potential hematologic, dermatologic, hypersensitivity, or hepatic reactions. These symptoms may include, but are not limited to, fever, sore throat, rash, ulcers in the mouth, easy bruising, lymphadenopathy, facial swelling, and petechial or purpuric hemorrhage, and in the case of liver reactions, anorexia, nausea/vomiting, or jaundice. Advise the patient that, because these signs and symptoms may signal a serious reaction, that they must report any occurrence immediately to a physician. In addition, advise the patient that these signs and symptoms should be reported even if mild or when occurring after extended use [see Warnings and Precautions (5.4, 5.5, 5.6, 5.8, 5.9)].
Angioedema
Advise patients to discontinue CEREBYX and seek immediate medical care if they develop signs or symptoms of angioedema such as facial, perioral, or upper airway swelling [see Warnings and Precautions (5.7)].
Hyperglycemia
Advise patients that CEREBYX may cause an increase in blood glucose levels [see Warnings and Precautions (5.17)].
Effects of Alcohol Use and Other Drugs and Over-the-Counter Drug Interactions
Caution patients against the use of other drugs or alcoholic beverages without first seeking their physician's advice [see Drug Interactions (7.1, 7.2)].
Inform patients that certain over-the-counter medications (e.g., cimetidine and omeprazole), vitamins (e.g., folic acid), and herbal supplements (e.g., St. John's wort) can alter their phenytoin levels.
Use in Pregnancy
Inform pregnant women and women of childbearing potential that use of CEREBYX during pregnancy can cause fetal harm, including an increased risk for cleft lip and/or cleft palate (oral clefts), cardiac defects, dysmorphic skull and facial features, nail and digit hypoplasia, growth abnormalities (including microcephaly), and cognitive deficits. When appropriate, counsel pregnant women and women of childbearing potential about alternative therapeutic options. Advise women of childbearing potential who are not planning a pregnancy to use effective contraception while using CEREBYX, keeping in mind that there is a potential for decreased hormonal contraceptive efficacy [see Drug Interactions (7.2)].
Instruct patients to notify their physician if they become pregnant or intend to become pregnant during therapy, and to notify their physician if they are breastfeeding or intend to breastfeed during therapy [see Use in Specific Populations (8.1, 8.2)].
Encourage patients to enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry if they become pregnant. This registry is collecting information about the safety of antiepileptic drugs during pregnancy [see Use in Specific Populations (8.1)].
- SPL UNCLASSIFIED SECTION
-
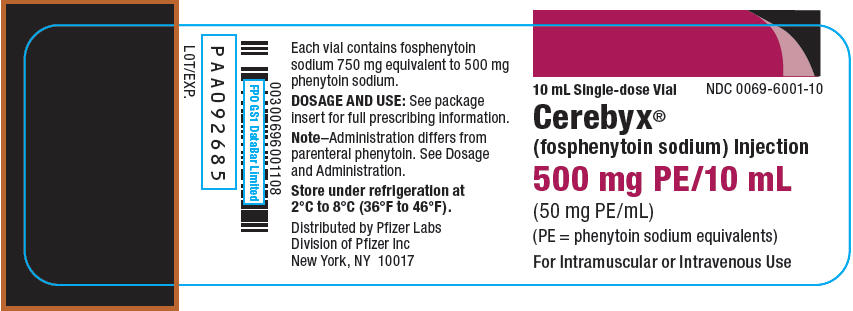
PRINCIPAL DISPLAY PANEL - 10 mL Vial Label
10 mL Single-dose Vial
NDC: 0069-6001-10Cerebyx®
(fosphenytoin sodium) Injection500 mg PE/10 mL
(50 mg PE/mL)
(PE = phenytoin sodium equivalents)For Intramuscular or Intravenous Use
-
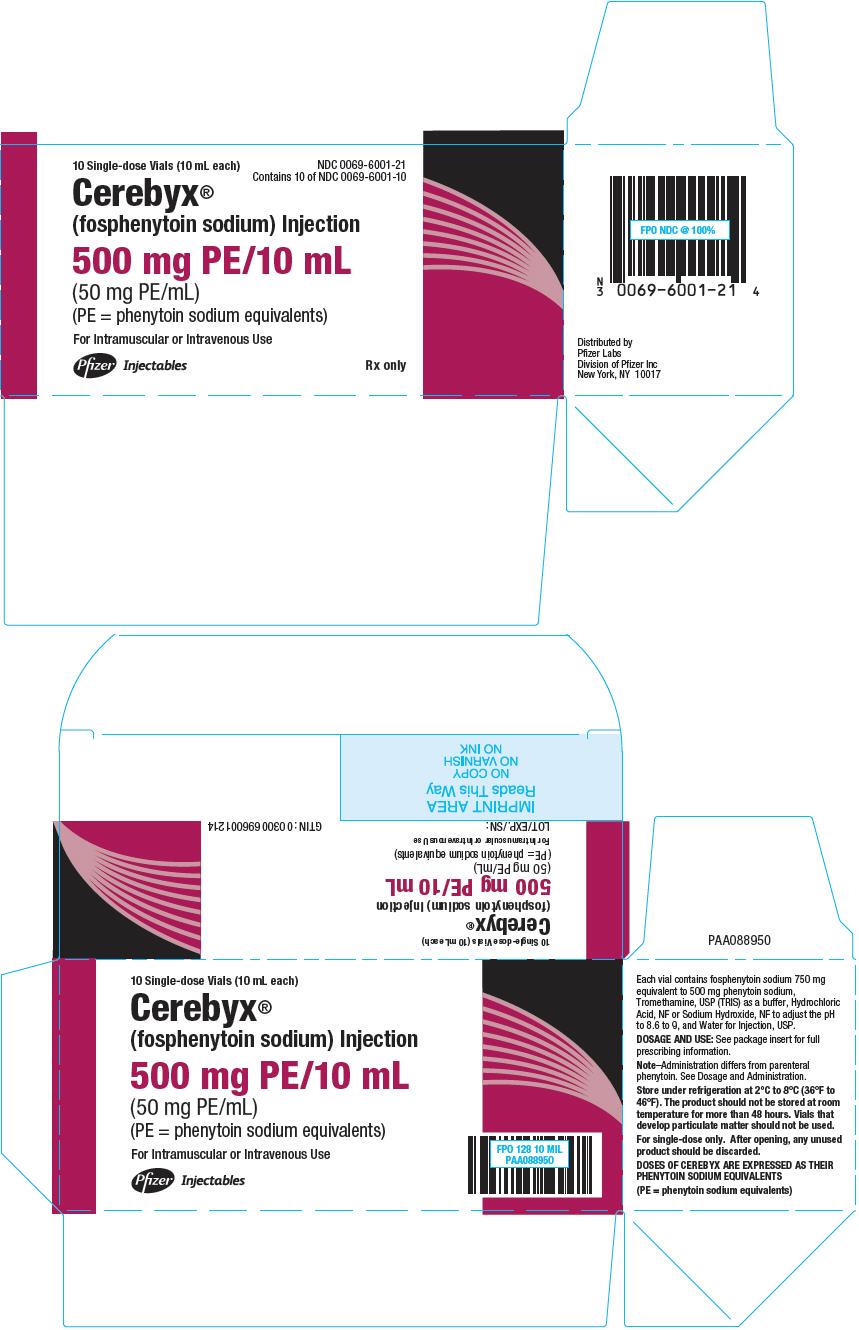
PRINCIPAL DISPLAY PANEL - 10 mL Vial Package
10 Single-dose Vials (10 mL each)
NDC: 0069-6001-21
Contains 10 of NDC: 0069-6001-10Cerebyx®
(fosphenytoin sodium) Injection500 mg PE/10 mL
(50 mg PE/mL)
(PE = phenytoin sodium equivalents)For Intramuscular or Intravenous Use
Pfizer Injectables
Rx only
-
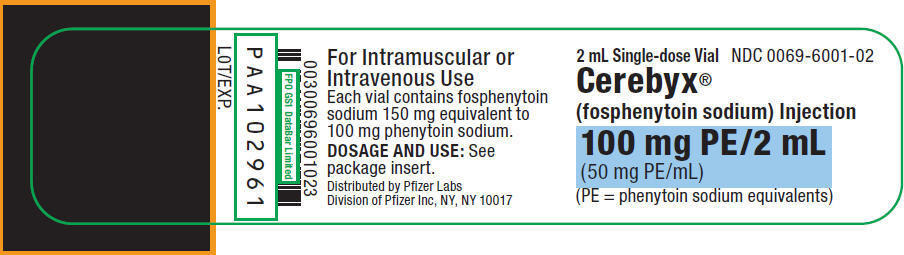
PRINCIPAL DISPLAY PANEL - 2 mL Vial Label
2 mL Single-dose Vial
NDC: 0069-6001-02Cerebyx®
(fosphenytoin sodium) Injection100 mg PE/2 mL
(50 mg PE/mL)(PE = phenytoin sodium equivalents)
-
PRINCIPAL DISPLAY PANEL - 2 mL Vial Carton
25 Single-dose Vials (2 mL each)
NDC: 0069-6001-25
Contains 25 of NDC: 0069-6001-02Cerebyx®
(fosphenytoin sodium) Injection100 mg PE/2 mL
(50 mg PE/mL)(PE = phenytoin sodium equivalents)
For Intramuscular or Intravenous Use
Pfizer Injectables
Rx only

-
INGREDIENTS AND APPEARANCE
CEREBYX
fosphenytoin sodium injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0069-6001 Route of Administration INTRAVENOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FOSPHENYTOIN SODIUM (UNII: 7VLR55452Z) (PHENYTOIN - UNII:6158TKW0C5) PHENYTOIN SODIUM 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) TROMETHAMINE (UNII: 023C2WHX2V) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0069-6001-21 10 in 1 PACKAGE 10/28/2013 1 NDC: 0069-6001-10 10 mL in 1 VIAL; Type 0: Not a Combination Product 2 NDC: 0069-6001-25 25 in 1 CARTON 10/28/2013 2 NDC: 0069-6001-02 2 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020450 10/28/2013 Labeler - Pfizer Laboratories Div Pfizer Inc (134489525) Registrant - Pfizer Inc (113480771) Establishment Name Address ID/FEI Business Operations Pharmacia and Upjohn Company LLC 618054084 ANALYSIS(0069-6001) , API MANUFACTURE(0069-6001) , LABEL(0069-6001) , MANUFACTURE(0069-6001) , PACK(0069-6001)
Trademark Results [CEREBYX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CEREBYX 75047723 2135222 Live/Registered |
WARNER-LAMBERT COMPANY LLC 1996-01-19 |
 CEREBYX 73800267 1578580 Dead/Cancelled |
WARNER-LAMBERT COMPANY 1989-05-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
