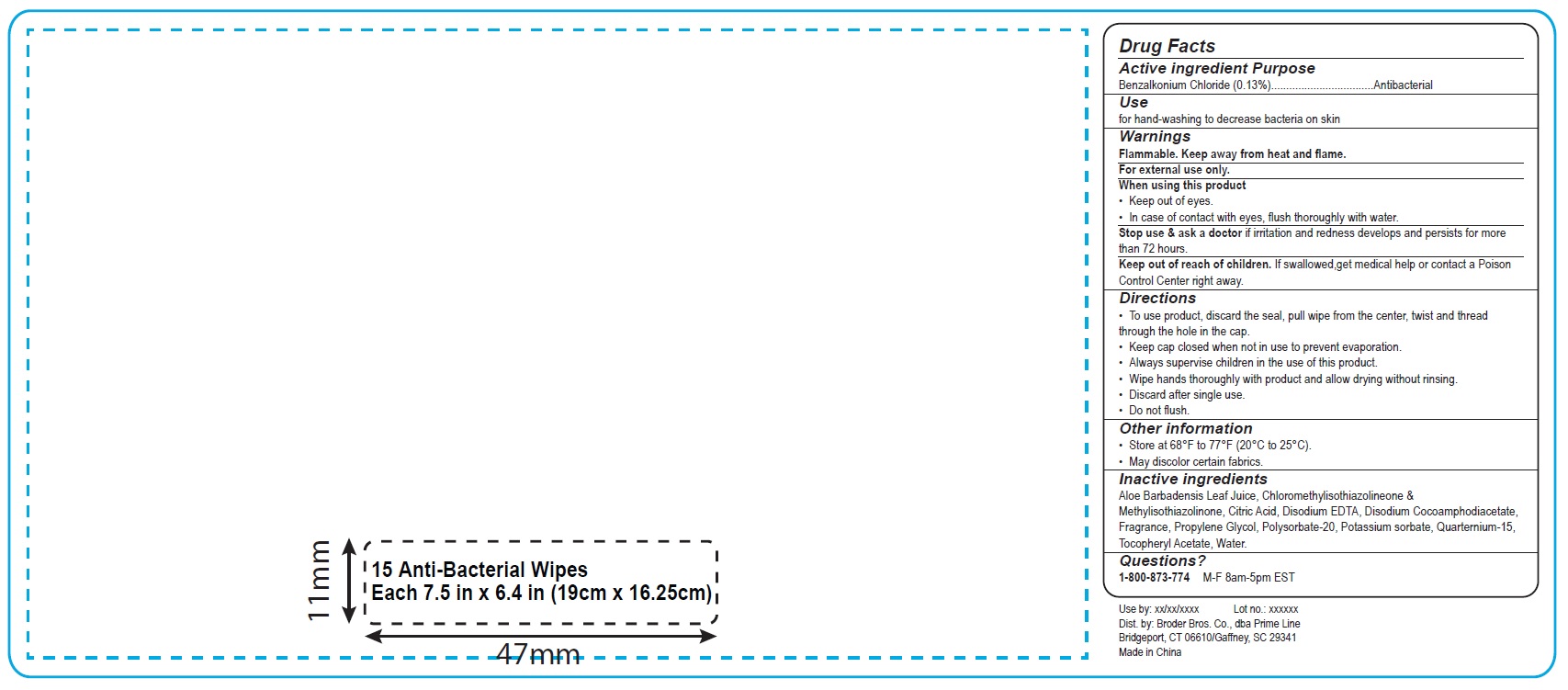
Antibacterial Wipes PC180 PC190 PL1801 PL1802 PL1803 PL1851
Antibacterial Wipes by
Drug Labeling and Warnings
Antibacterial Wipes by is a Otc medication manufactured, distributed, or labeled by Broder Bros Prime Line. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ANTIBACTERIAL WIPES- benzalkonium chloride cloth
Broder Bros Prime Line
----------
Antibacterial Wipes PC180 PC190 PL1801 PL1802 PL1803 PL1851
Warnings
Flammable. Keep away from heat and flame.
For external use only
Directions
- To use product, discard the seal, pull wipe from the center, twist and thread through the hole in the cap.
- Keep cap closed when not in use to prevent evaporation.
- Always supervise children in the use of this product.
- Wipe hands thoroughly with product and alow drying without rinsing.
- Discard after single use.
- Do not flush.
Inactive ingredients
Aloe Barbadensis Leaf Juice, Chloromethylisothiazolinone& Methylisothiazolinone, Citric Acid,
Disodium Cocoamphodiacetate, Disodium EDTA, Fragrance, Polysorbate-20, Potassium Sorbate,
Propylene Glycol, Quaternium-15, Tocopheryl Acetate, Water.
| ANTIBACTERIAL WIPES
benzalkonium chloride cloth |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Broder Bros Prime Line (107044246) |
Revised: 11/2023
Document Id: 09ae111c-9389-a8f2-e063-6294a90a1539
Set id: d4c855c2-4c07-f2a4-e053-2995a90a8cf0
Version: 3
Effective Time: 20231108
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.