PIPERACILLIN, TAZOBACTAM- piperacillin sodium, tazobactam sodium injection, powder, lyophilized, for solution
Piperacillin, Tazobactam by
Drug Labeling and Warnings
Piperacillin, Tazobactam by is a Prescription medication manufactured, distributed, or labeled by Sagent Pharmaceuticals. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PIPERACILLIN AND TAZOBACTAM FOR INJECTION safely and effectively. See full prescribing information for PIPERACILLIN AND TAZOBACTAM FOR INJECTION.
PIPERACILLIN and TAZOBACTAM for injection, for intravenous use
Initial U.S. approval: 1993INDICATIONS AND USAGE
Piperacillin and tazobactam for injection is a combination penicillin-class antibacterial and β-lactamase inhibitor indicated for treatment of:
- Intra-abdominal infections (1.1)
- Skin and skin structure infections (1.2)
- Female pelvic infections (1.3)
- Community-acquired pneumonia (1.4)
- Nosocomial pneumonia (1.5)
- Usage (1.6)
To reduce the development of drug-resistant bacteria and maintain the effectiveness of piperacillin and tazobactam for injection and other antibacterial drugs, piperacillin and tazobactam for injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. (1.6)
DOSAGE AND ADMINISTRATION
- The usual daily dose of piperacillin and tazobactam for injection for adults is 3.375 grams every six hours totaling 13.5 grams (12 grams piperacillin/1.5 grams tazobactam) (2.1)
- Initial presumptive treatment of patients with nosocomial pneumonia should start with piperacillin and tazobactam for injection at a dosage of 4.5 grams every six hours plus an aminoglycoside, totaling 18 grams (16 grams piperacillin/2 grams tazobactam). (2.2)
- Dosage in patients with renal impairment (≤40 mL/min of CRCL) and dialysis patients should be reduced, based on the degree of actual renal function impairment. (2.3)
- For children with appendicitis and/or peritonitis the recommended piperacillin and tazobactam for injection dosage is 100 mg piperacillin/12.5 mg tazobactam per kilogram of body weight, every 8 hours in pediatric patients 9 months of age and older. For pediatric patients 2 to 9 months of age, the recommended dosage is 80 mg piperacillin/10 mg tazobactam per kilogram of body weight, every 8 hours. (2.4)
- Piperacillin and tazobactam for injection and aminoglycosides should be reconstituted, diluted, and administered separately. Co-administration via Y-site can be done under certain conditions. (2.7)
DOSAGE FORMS AND STRENGTHS
Piperacillin and Tazobactam for Injection: 2.25 gram, 3.375 gram, and 4.5 gram lyophilized powder for reconstitution in single-dose vials. (3)
CONTRAINDICATIONS
Patients with a history of allergic reactions to any of the penicillins, cephalosporins, or β-lactamase inhibitors. (4)
WARNINGS AND PRECAUTIONS
- Serious hypersensitivity reactions (anaphylactic/anaphylactoid) reactions have been reported in patients receiving piperacillin and tazobactam. Discontinue piperacillin and tazobactam if a reaction occurs. (5.1)
- Piperacillin and tazobactam may cause severe cutaneous adverse reactions, such as Stevens-Johnson syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms, and acute generalized exanthematous pustulosis (5.2). Discontinue piperacillin and tazobactam for progressive rashes.
- Hematological effects (including bleeding, leukopenia and neutropenia) have occurred. Monitor hematologic tests during prolonged therapy. (5.3)
- Nephrotoxicity in critically ill patients has been observed; the use of piperacillin and tazobactam was found to be an independent risk factor for renal failure and was associated with delayed recovery of renal function as compared to other beta-lactam antibacterial drugs in a randomized, multicenter, controlled trial in critically ill patients. Based on this study, alternative treatment options should be considered in the critically ill population. If alternative treatment options are inadequate or unavailable, monitor renal function during treatment with Piperacillin and tazobactam. (5.5)
- Clostridium difficile associated diarrhea: evaluate patients if diarrhea occurs. (5.7)
ADVERSE REACTIONS
The most common adverse reactions (incidence >5%) are diarrhea, constipation, nausea, headache and insomnia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Sagent Pharmaceuticals, Inc. at 1-866-625-1618 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Piperacillin and tazobactam administration can significantly reduce tobramycin concentrations in hemodialysis patients. Monitor tobramycin concentrations in these patients. (7.1)
- Probenecid prolongs the half-lives of piperacillin and tazobactam and should not be co-administered with piperacillin and tazobactam unless the benefit outweighs the risk. (7.2)
- Co-administration of piperacillin and tazobactam with vancomycin may increase the incidence of acute kidney injury. Monitor kidney function in patients receiving piperacillin and tazobactam and vancomycin. (7.3)
- Monitor coagulation parameters in patients receiving piperacillin and tazobactam and heparin or oral anticoagulants. (7.4)
- Piperacillin and tazobactam may prolong the neuromuscular blockade of vecuronium and other non-depolarizing muscle relaxants. Monitor for adverse reactions related to neuromuscular blockade (7.5)
USE IN SPECIFIC POPULATIONS
Dosage in patients with renal impairment (≤40 mL/min of CRCL) should be reduced to the degree of actual renal function impairment. (2.3, 8.6)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Intra-abdominal Infections
1.2 Skin and Skin Structure Infections
1.3 Female Pelvic Infections
1.4 Community-acquired Pneumonia
1.5 Nosocomial Pneumonia
1.6 Usage
2 DOSAGE AND ADMINISTRATION
2.1 Adult Patients
2.2 Nosocomial Pneumonia
2.3 Renal Impairment
2.4 Pediatric Patients
2.5 Reconstitution and Dilution of Powder Formulations
2.7 Compatibility with Aminoglycosides
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Adverse Reactions
5.2 Severe Cutaneous Adverse Reactions
5.3 Hematologic Adverse Reactions
5.4 Central Nervous System Adverse Reactions
5.5 Nephrotoxicity in Critically Ill Patients
5.6 Electrolyte Effects
5.7 Clostridium difficile Associated Diarrhea
5.8 Development of Drug-Resistant Bacteria
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-Marketing Experience
6.3 Additional Experience with piperacillin
7 DRUG INTERACTIONS
7.1 Aminoglycosides
7.2 Probenecid
7.3 Vancomycin
7.4 Anticoagulants
7.5 Vecuronium
7.6 Methotrexate
7.7 Effects on Laboratory Tests
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
8.8 Patients with Cystic Fibrosis
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
SPL UNCLASSIFIED SECTION
To reduce the development of drug-resistant bacteria and maintain the effectiveness of piperacillin and tazobactam for injection and other antibacterial drugs, piperacillin and tazobactam for injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
-
1 INDICATIONS AND USAGE
Piperacillin and tazobactam for injection is a combination product consisting of a penicillin-class antibacterial, piperacillin, and a β-lactamase inhibitor, tazobactam, indicated for the treatment of patients with moderate to severe infections caused by susceptible isolates of the designated bacteria in the conditions listed below.
1.1 Intra-abdominal Infections
Appendicitis (complicated by rupture or abscess) and peritonitis caused by β-lactamase producing isolates of Escherichia coli or the following members of the Bacteroides fragilis group: B. fragilis, B. ovatus, B. thetaiotaomicron, or B. vulgatus. The individual members of this group were studied in fewer than 10 cases.
1.2 Skin and Skin Structure Infections
Uncomplicated and complicated skin and skin structure infections, including cellulitis, cutaneous abscesses and ischemic/diabetic foot infections caused by β-lactamase producing isolates of Staphylococcus aureus.
1.3 Female Pelvic Infections
Postpartum endometritis or pelvic inflammatory disease caused by β-lactamase producing isolates of Escherichia coli.
1.4 Community-acquired Pneumonia
Community-acquired pneumonia (moderate severity only) caused by β-lactamase producing isolates of Haemophilus influenzae.
1.5 Nosocomial Pneumonia
Nosocomial pneumonia (moderate to severe) caused by β-lactamase producing isolates of Staphylococcus aureus and by piperacillin/tazobactam-susceptible Acinetobacter baumannii, Haemophilus influenzae, Klebsiella pneumoniae, and Pseudomonas aeruginosa (Nosocomial pneumonia caused by P. aeruginosa should be treated in combination with an aminoglycoside) [see Dosage and Administration (2)].
1.6 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of piperacillin and tazobactam for injection and other antibacterial drugs, piperacillin and tazobactam for injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
-
2 DOSAGE AND ADMINISTRATION
Piperacillin and tazobactam for injection should be administered by intravenous infusion over 30 minutes.
2.1 Adult Patients
The usual total daily dose of piperacillin and tazobactam for injection for adults is 3.375 grams every six hours totaling 13.5 grams (12 grams piperacillin/1.5 grams tazobactam). The usual duration of piperacillin and tazobactam for injection treatment is from 7 to 10 days.
Piperacillin and tazobactam for injection should be administered by intravenous infusion over 30 minutes.
2.2 Nosocomial Pneumonia
Initial presumptive treatment of patients with nosocomial pneumonia should start with piperacillin and tazobactam for injection at a dosage of 4.5 grams every six hours plus an aminoglycoside, totaling 18 grams (16 grams piperacillin/2 grams tazobactam). The recommended duration of piperacillin and tazobactam for injection treatment for nosocomial pneumonia is 7 to 14 days. Treatment with the aminoglycoside should be continued in patients from whom P. aeruginosa is isolated.
2.3 Renal Impairment
In patients with renal impairment (creatinine clearance ≤40 mL/min) and dialysis patients (hemodialysis and CAPD), the intravenous dose of piperacillin and tazobactam for injection should be reduced to the degree of actual renal function impairment. The recommended daily doses of piperacillin and tazobactam for injection for patients with renal impairment are as follows:
Table 1: Recommended Dosing of Piperacillin and Tazobactam for Injection in Patients with Normal Renal Function and Renal-Impairment (As total grams piperacillin/tazobactam) * Creatinine clearance for patients not receiving hemodialysis
** 0.75 gram (0.67 gram piperacillin/0.08 gram tazobactam) should be administered following each hemodialysis session on hemodialysis days
Renal Function
(creatinine clearance, mL/min)All Indications
(except nosocomial pneumonia)Nosocomial
Pneumonia>40 mL/min 3.375 q 6 h 4.5 q 6 h 20 to 40 mL/min* 2.25 q 6 h 3.375 q 6 h <20 mL/min* 2.25 q 8 h 2.25 q 6 h Hemodialysis** 2.25 q 12 h 2.25 q 8 h CAPD 2.25 q 12 h 2.25 q 8 h For patients on hemodialysis, the maximum dose is 2.25 grams every twelve hours for all indications other than nosocomial pneumonia and 2.25 grams every eight hours for nosocomial pneumonia. Since hemodialysis removes 30% to 40% of the administered dose, an additional dose of 0.75 gram piperacillin and tazobactam for injection (0.67 gram piperacillin/0.08 gram tazobactam) should be administered following each dialysis period on hemodialysis days. No additional dosage of piperacillin and tazobactam for injection is necessary for CAPD patients.
2.4 Pediatric Patients
For children with appendicitis and/or peritonitis 9 months of age or older, weighing up to 40 kg, and with normal renal function, the recommended piperacillin and tazobactam for injection dosage is 100 mg piperacillin/12.5 mg tazobactam per kilogram of body weight, every 8 hours. For pediatric patients between 2 months and 9 months of age, the recommended piperacillin and tazobactam for injection dosage based on pharmacokinetic modeling, is 80 mg piperacillin/10 mg tazobactam per kilogram of body weight, every 8 hours [see Use in Specific Populations (8.4) and Clinical Pharmacology (12.3)]. Pediatric patients weighing over 40 kg and with normal renal function should receive the adult dose.
It has not been determined how to adjust piperacillin and tazobactam for injection dosage in pediatric patients with renal impairment.
2.5 Reconstitution and Dilution of Powder Formulations
Single dose vials
Reconstitute piperacillin and tazobactam for injection vials with a compatible reconstitution diluent from the list provided below.
2.25 gram, 3.375 gram, and 4.5 gram piperacillin and tazobactam for injection should be reconstituted with 10 mL, 15 mL, and 20 mL, respectively. Swirl until dissolved.
Compatible Reconstitution Diluents for Single Dose Vials
0.9% sodium chloride for injection
Sterile water for injection
Dextrose 5%
Bacteriostatic saline/parabens
Bacteriostatic water/parabens
Bacteriostatic saline/benzyl alcohol
Bacteriostatic water/benzyl alcoholReconstituted piperacillin and tazobactam for injection solutions for single dose vials should be further diluted (recommended volume per dose of 50 mL to 150 mL) in a compatible intravenous solution listed below. Administer by infusion over a period of at least 30 minutes. During the infusion it is desirable to discontinue the primary infusion solution.
Compatible Intravenous Solutions for Single Dose Vials
0.9% sodium chloride for injection
sterile water for injection‡
Dextran 6% in saline
Dextrose 5%LACTATED RINGER'S SOLUTION IS NOT COMPATIBLE WITH PIPERACILLIN AND TAZOBACTAM FOR INJECTION.
‡ Maximum recommended volume per dose of sterile water for injection is 50 mL.
Piperacillin and tazobactam should not be mixed with other drugs in a syringe or infusion bottle since compatibility has not been established.
Piperacillin and tazobactam is not chemically stable in solutions that contain only sodium bicarbonate and solutions that significantly alter the pH.
Piperacillin and tazobactam should not be added to blood products or albumin hydrolysates. Parenteral drug products should be inspected visually for particulate matter or discoloration prior to administration, whenever solution and container permit.
Stability of Piperacillin and Tazobactam Powder Formulations Following Reconstitution
Piperacillin and tazobactam for injection reconstituted from single dose vials is stable in glass and plastic containers (plastic syringes, IV bags and tubing) when used with compatible diluents.
Single dose vials should be used immediately after reconstitution. Discard any unused portion after 24 hours if stored at room temperature (20° to 25°C [68° to 77°F]), or after 48 hours if stored at refrigerated temperature (2° to 8°C [36° to 46°F]). Vials should not be frozen after reconstitution.
Stability studies in the IV bags have demonstrated chemical stability (potency, pH of reconstituted solution and clarity of solution) for up to 24 hours at room temperature and up to one week at refrigerated temperature. Piperacillin and tazobactam for injection contains no preservatives. Appropriate consideration of aseptic technique should be used.
Piperacillin and tazobactam for injection reconstituted from single dose vials can be used in ambulatory intravenous infusion pumps. Stability of piperacillin and tazobactam for injection in an ambulatory intravenous infusion pump has been demonstrated for a period of 12 hours at room temperature. Each dose was reconstituted and diluted to a volume of 37.5 mL or 25 mL. One-day supplies of dosing solution were aseptically transferred into the medication reservoir (IV bags or cartridge). The reservoir was fitted to a preprogrammed ambulatory intravenous infusion pump per the manufacturer's instructions. Stability of piperacillin and tazobactam for injection is not affected when administered using an ambulatory intravenous infusion pump.
2.7 Compatibility with Aminoglycosides
Due to the in vitro inactivation of aminoglycosides by piperacillin, piperacillin and tazobactam and aminoglycosides are recommended for separate administration. Piperacillin and tazobactam and aminoglycosides should be reconstituted, diluted, and administered separately when concomitant therapy with aminoglycosides is indicated [see Drug Interactions (7.1)].
In circumstances where co-administration via Y-site is necessary, piperacillin and tazobactam is compatible for simultaneous co-administration via Y-site infusion only with the following aminoglycosides under the following conditions:
Table 2: Compatibility with Aminoglycosides a Diluent volumes apply only to single dose vials
b The concentration ranges in Table 2 are based on administration of the aminoglycoside in divided doses (10 to 15 mg/kg/day in two daily doses for amikacin and 3 to 5 mg/kg/day in three daily doses for gentamicin). Administration of amikacin or gentamicin in a single daily dose or in doses exceeding those stated above via Y-site with piperacillin and tazobactam has not been evaluated. See package insert for each aminoglycoside for complete Dosage and Administration instructions.
Aminoglycoside Piperacillin and Tazobactam Dose
(grams)Piperacillin and Tazobactam Diluent Volumea
(mL)Aminoglycoside
Concentration
Rangeb
(mg/mL)Acceptable Diluents Amikacin 2.25
3.375
4.550
100
1501.75 to 7.5 0.9% sodium chloride
or 5% dextroseGentamicin 2.25
3.375
4.550
100
1500.7 to 3.32 0.9% sodium chloride
or 5% dextroseOnly the concentration and diluents for amikacin or gentamicin with the dosages of piperacillin and tazobactam listed above have been established as compatible for co-administration via Y-site infusion. Simultaneous co-administration via Y-site infusion in any manner other than listed above may result in inactivation of the aminoglycoside by piperacillin and tazobactam.
Piperacillin and tazobactam is not compatible with tobramycin for simultaneous co-administration via Y-site infusion. Compatibility of piperacillin and tazobactam for injection with other aminoglycosides has not been established.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
-
3 DOSAGE FORMS AND STRENGTHS
Piperacillin and Tazobactam for Injection, USP is supplied as a white to off-white sterile, cryodesiccated powder in vials of the following sizes:
Each piperacillin and tazobactam for injection, USP 2.25 gram vial provides piperacillin sodium equivalent to 2 grams of piperacillin and tazobactam sodium equivalent to 0.25 gram of tazobactam. Each vial contains 4.7 mEq (108 mg) of sodium.
Each piperacillin and tazobactam for injection, USP 3.375 gram vial provides piperacillin sodium equivalent to 3 grams of piperacillin and tazobactam sodium equivalent to 0.375 gram of tazobactam. Each vial contains 7.05 mEq (162 mg) of sodium.
Each piperacillin and tazobactam for injection, USP 4.5 gram vial provides piperacillin sodium equivalent to 4 grams of piperacillin and tazobactam sodium equivalent to 0.5 gram of tazobactam. Each vial contains 9.4 mEq (216 mg) of sodium.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Adverse Reactions
Serious and occasionally fatal hypersensitivity (anaphylactic/anaphylactoid) reactions (including shock) have been reported in patients receiving therapy with piperacillin and tazobactam. These reactions are more likely to occur in individuals with a history of penicillin, cephalosporin, or carbapenem hypersensitivity or a history of sensitivity to multiple allergens. Before initiating therapy with piperacillin and tazobactam, careful inquiry should be made concerning previous hypersensitivity reactions. If an allergic reaction occurs, piperacillin and tazobactam should be discontinued and appropriate therapy instituted.
5.2 Severe Cutaneous Adverse Reactions
Piperacillin and tazobactam may cause severe cutaneous adverse reactions, such as Stevens-Johnson syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms, and acute generalized exanthematous pustulosis. If patients develop a skin rash they should be monitored closely and piperacillin and tazobactam discontinued if lesions progress.
5.3 Hematologic Adverse Reactions
Bleeding manifestations have occurred in some patients receiving β-lactam drugs, including piperacillin. These reactions have sometimes been associated with abnormalities of coagulation tests such as clotting time, platelet aggregation and prothrombin time, and are more likely to occur in patients with renal failure. If bleeding manifestations occur, piperacillin and tazobactam should be discontinued and appropriate therapy instituted.
The leukopenia/neutropenia associated with piperacillin and tazobactam administration appears to be reversible and most frequently associated with prolonged administration.
Periodic assessment of hematopoietic function should be performed, especially with prolonged therapy, i.e., ≥ 21 days [see Adverse Reactions (6.1)].
5.4 Central Nervous System Adverse Reactions
As with other penicillins, patients may experience neuromuscular excitability or convulsions if higher than recommended doses are given intravenously (particularly in the presence of renal failure).
5.5 Nephrotoxicity in Critically Ill Patients
The use of piperacillin and tazobactam was found to be an independent risk factor for renal failure and was associated with delayed recovery of renal function as compared to other beta-lactam antibacterial drugs in a randomized, multicenter, controlled trial in critically ill patients [see Adverse Reactions (6.1)]. Based on this study, alternative treatment options should be considered in the critically ill population. If alternative treatment options are inadequate or unavailable, monitor renal function during treatment with piperacillin and tazobactam [see Dosage and Administration (2.3)].
Combined use of piperacillin/tazobactam and vancomycin may be associated with an increased incidence of acute kidney injury [see Drug Interactions (7.3)].
5.6 Electrolyte Effects
Piperacillin and tazobactam for injection contains a total of 2.35 mEq (54 mg) of Na+ (sodium) per gram of piperacillin in the combination product. This should be considered when treating patients requiring restricted salt intake. Periodic electrolyte determinations should be performed in patients with low potassium reserves, and the possibility of hypokalemia should be kept in mind with patients who have potentially low potassium reserves and who are receiving cytotoxic therapy or diuretics.
5.7 Clostridium difficile Associated Diarrhea
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including piperacillin and tazobactam, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial drug use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial drug use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
During the initial clinical investigations, 2621 patients worldwide were treated with piperacillin and tazobactam for injection in phase 3 trials. In the key North American monotherapy clinical trials (n=830 patients), 90% of the adverse events reported were mild to moderate in severity and transient in nature. However, in 3.2% of the patients treated worldwide, piperacillin and tazobactam was discontinued because of adverse events primarily involving the skin (1.3%), including rash and pruritus; the gastrointestinal system (0.9%), including diarrhea, nausea, and vomiting; and allergic reactions (0.5%).
Table 3: Adverse Reactions from Piperacillin and Tazobactam for Injection Monotherapy Clinical Trials System Organ Class
Adverse ReactionGastrointestinal disorders
Diarrhea (11.3%)
Constipation (7.7%)
Nausea (6.9%)
Vomiting (3.3%)
Dyspepsia (3.3%)
Abdominal pain (1.3%)General disorders and administration site conditions
Fever (2.4%)
Injection site reaction (≤1%)
Rigors (≤1%)Immune system disorders
Anaphylaxis (≤1%)Infections and infestations
Candidiasis (1.6%)
Pseudomembranous colitis (≤1%)Metabolism and nutrition disorders
Hypoglycemia (≤1%)Musculoskeletal and connective tissue disorders
Myalgia (≤1%)
Arthralgia (≤1%)Nervous system disorders
Headache (7.7%)Psychiatric disorders
Insomnia (6.6%)Skin and subcutaneous tissue disorders
Rash (4.2%, including maculopapular, bullous, and urticarial)
Pruritus (3.1%)
Purpura (≤1%)Vascular disorders
Phlebitis (1.3%)
Thrombophlebitis (≤1%)
Hypotension (≤1%)
Flushing (≤1%)Respiratory, thoracic and mediastinal disorders
Epistaxis (≤1%)Nosocomial Pneumonia Trials
Two trials of nosocomial lower respiratory tract infections were conducted. In one study, 222 patients were treated with piperacillin and tazobactam in a dosing regimen of 4.5 g every 6 hours in combination with an aminoglycoside and 215 patients were treated with imipenem/cilastatin (500 mg/500 mg q6h) in combination with an aminoglycoside. In this trial, treatment-emergent adverse events were reported by 402 patients, 204 (91.9%) in the piperacillin/tazobactam group and 198 (92.1%) in the imipenem/cilastatin group. Twenty-five (11.0%) patients in the piperacillin/tazobactam group and 14 (6.5%) in the imipenem/cilastatin group (p > 0.05) discontinued treatment due to an adverse event.
The second trial used a dosing regimen of 3.375 g given every 4 hours with an aminoglycoside.
Table 4: Adverse Reactions from Piperacillin and Tazobactam for Injection Plus Aminoglycoside Clinical Trialsa a For adverse drug reactions that appeared in both studies the higher frequency is presented.
System Organ Class
Adverse ReactionBlood and lymphatic system disorders
Thrombocythemia (1.4%)
Anemia (≤1%)
Thrombocytopenia (≤1%)
Eosinophilia (≤1%)Gastrointestinal disorders
Diarrhea (20%)
Constipation (8.4%)
Nausea (5.8%)
Vomiting (2.7%)
Dyspepsia (1.9%)
Abdominal pain (1.8%)
Stomatitis (≤1%)General disorders and administration site conditions
Fever (3.2%)
Injection site reaction (≤1%)Infections and infestations
Oral candidiasis (3.9%)
Candidiasis (1.8%)Investigations
BUN increased (1.8%)
Blood creatinine increased (1.8%)
Liver function test abnormal (1.4%)
Alkaline phosphatase increased (≤1%)
Aspartate aminotransferase increased (≤1%)
Alanine aminotransferase increased (≤1%)Metabolism and nutrition disorders
Hypoglycemia (≤1%)
Hypokalemia (≤1%)Nervous system disorders
Headache (4.5%)Psychiatric disorders
Insomnia (4.5%)Renal and urinary disorders
Renal failure (≤1%)Skin and subcutaneous tissue disorders
Rash (3.9%)
Pruritus (3.2%)Vascular disorders
Thrombophlebitis (1.3%)
Hypotension (1.3%)Other trials: Nephrotoxicity
In a randomized, multicenter, controlled trial in 1200 adult critically ill patients, piperacillin/tazobactam was found to be a risk factor for renal failure (odds ratio 1.7, 95% CI 1.18 to 2.43), and associated with delayed recovery of renal function as compared to other beta-lactam antibacterial drugs.1 [see Warnings and Precautions (5.5)].
Pediatrics
Studies of piperacillin and tazobactam in pediatric patients suggest a similar safety profile to that seen in adults. In a prospective, randomized, comparative, open-label clinical trial of pediatric patients with severe intra-abdominal infections (including appendicitis and/or peritonitis), 273 patients were treated with piperacillin and tazobactam (112.5 mg/kg every 8 hours) and 269 patients were treated with cefotaxime (50 mg/kg) plus metronidazole (7.5 mg/kg) every 8 hours. In this trial, treatment-emergent adverse events were reported by 146 patients, 73 (26.7%) in the piperacillin and tazobactam group and 73 (27.1%) in the cefotaxime/metronidazole group. Six patients (2.2%) in the piperacillin and tazobactam group and 5 patients (1.9%) in the cefotaxime/metronidazole group discontinued due to an adverse event.
Adverse Laboratory Events (Seen During Clinical Trials)
Of the trials reported, including that of nosocomial lower respiratory tract infections in which a higher dose of piperacillin and tazobactam for injection was used in combination with an aminoglycoside, changes in laboratory parameters include:
Hematologic—decreases in hemoglobin and hematocrit, thrombocytopenia, increases in platelet count, eosinophilia, leukopenia, neutropenia. These patients were withdrawn from therapy; some had accompanying systemic symptoms (e.g., fever, rigors, chills)
Coagulation—positive direct Coombs' test, prolonged prothrombin time, prolonged partial thromboplastin time
Hepatic—transient elevations of AST (SGOT), ALT (SGPT), alkaline phosphatase, bilirubin
Renal—increases in serum creatinine, blood urea nitrogen
Additional laboratory events include abnormalities in electrolytes (i.e., increases and decreases in sodium, potassium, and calcium), hyperglycemia, decreases in total protein or albumin, blood glucose decreased, gamma-glutamyltransferase increased, hypokalemia, and bleeding time prolonged.
6.2 Post-Marketing Experience
In addition to the adverse drug reactions identified in clinical trials in Table 3 and Table 4, the following adverse reactions have been identified during post-approval use of piperacillin and tazobactam.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hepatobiliary—hepatitis, jaundice
Hematologic—hemolytic anemia, agranulocytosis, pancytopenia
Immune—hypersensitivity reactions, anaphylactic/anaphylactoid reactions (including shock)
Renal—interstitial nephritis
Respiratory—eosinophilic pneumonia
Skin and Appendages—erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms (DRESS), acute generalized exanthematous pustulosis (AGEP), dermatitis exfoliative
6.3 Additional Experience with piperacillin
The following adverse reaction has also been reported for piperacillin for injection:
Skeletal—prolonged muscle relaxation [see Drug Interactions (7.5)].
Post-marketing experience with piperacillin and tazobactam in pediatric patients suggests a similar safety profile to that seen in adults.
-
7 DRUG INTERACTIONS
7.1 Aminoglycosides
Piperacillin may inactivate aminoglycosides by converting them to microbiologically inert amides.
In vivo inactivation:
When aminoglycosides are administered in conjunction with piperacillin to patients with end-stage renal disease requiring hemodialysis, the concentrations of the aminoglycosides (especially tobramycin) may be significantly reduced and should be monitored.
Sequential administration of piperacillin and tazobactam and tobramycin to patients with either normal renal function or mild to moderate renal impairment has been shown to modestly decrease serum concentrations of tobramycin but no dosage adjustment is considered necessary.
In vitro inactivation:
Due to the in vitro inactivation of aminoglycosides by piperacillin, piperacillin and tazobactam and aminoglycosides are recommended for separate administration. Piperacillin and tazobactam and aminoglycosides should be reconstituted, diluted, and administered separately when concomitant therapy with aminoglycosides is indicated. Piperacillin and tazobactam is compatible with amikacin and gentamicin for simultaneous Y-site infusion in certain diluents and at specific concentrations. Piperacillin and tazobactam is not compatible with tobramycin for simultaneous Y-site infusion [see Dosage and Administration (2.7)].
7.2 Probenecid
Probenecid administered concomitantly with piperacillin and tazobactam prolongs the half-life of piperacillin by 21% and that of tazobactam by 71% because probenecid inhibits tubular renal secretion of both piperacillin and tazobactam. Probenecid should not be co-administered with piperacillin and tazobactam unless the benefit outweighs the risk.
7.3 Vancomycin
Studies have detected an increased incidence of acute kidney injury in patients concomitantly administered piperacillin/tazobactam and vancomycin as compared to vancomycin alone [see Warnings and Precautions (5.5)].
Monitor kidney function in patients concomitantly administered with piperacillin/tazobactam and vancomycin.
No pharmacokinetic interactions have been noted between piperacillin/tazobactam and vancomycin.
7.4 Anticoagulants
Coagulation parameters should be tested more frequently and monitored regularly during simultaneous administration of high doses of heparin, oral anticoagulants, or other drugs that may affect the blood coagulation system or the thrombocyte function [see Warnings and Precautions (5.3)].
7.5 Vecuronium
Piperacillin when used concomitantly with vecuronium has been implicated in the prolongation of the neuromuscular blockade of vecuronium. Piperacillin and tazobactam could produce the same phenomenon if given along with vecuronium. Due to their similar mechanism of action, it is expected that the neuromuscular blockade produced by any of the non-depolarizing muscle relaxants could be prolonged in the presence of piperacillin. Monitor for adverse reactions related to neuromuscular blockade (See package insert for vecuronium bromide).
7.6 Methotrexate
Limited data suggests that co-administration of methotrexate and piperacillin may reduce the clearance of methotrexate due to competition for renal secretion. The impact of tazobactam on the elimination of methotrexate has not been evaluated. If concurrent therapy is necessary, serum concentrations of methotrexate as well as the signs and symptoms of methotrexate toxicity should be frequently monitored.
7.7 Effects on Laboratory Tests
There have been reports of positive test results using the Bio-Rad Laboratories Platelia Aspergillus EIA test in patients receiving piperacillin/tazobactam injection who were subsequently found to be free of Aspergillus infection. Cross-reactions with non-Aspergillus polysaccharides and polyfuranoses with the Bio-Rad Laboratories Platelia Aspergillus EIA test have been reported. Therefore, positive test results in patients receiving piperacillin/tazobactam should be interpreted cautiously and confirmed by other diagnostic methods.
As with other penicillins, the administration of piperacillin and tazobactam for injection may result in a false-positive reaction for glucose in the urine using a copper-reduction method (CLINITEST®). It is recommended that glucose tests based on enzymatic glucose oxidase reactions be used.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Piperacillin and tazobactam cross the placenta in humans. However, there are insufficient data with piperacillin and/or tazobactam in pregnant women to inform a drug-associated risk for major birth defects and miscarriage. No fetal structural abnormalities were observed in rats or mice when piperacillin/tazobactam was administered intravenously during organogenesis at doses 1 to 2 times and 2 to 3 times the human dose of piperacillin and tazobactam, respectively, based on body-surface area (mg/m2). However, fetotoxicity in the presence of maternal toxicity was observed in developmental toxicity and peri/postnatal studies conducted in rats (intraperitoneal administration prior to mating and throughout gestation or from gestation day 17 through lactation day 21) at doses less than the maximum recommended human daily dose based on body-surface area (mg/m2) [see Data].
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
In embryo-fetal development studies in mice and rats, pregnant animals received intravenous doses of piperacillin/tazobactam up to 3,000/750 mg/kg/day during the period of organogenesis. There was no evidence of teratogenicity up to the highest dose evaluated, which is 1 to 2 times and 2 to 3 times the human dose of piperacillin and tazobactam, in mice and rats respectively, based on body-surface area (mg/m2). Fetal body weights were reduced in rats at maternally toxic doses at or above 500/62.5 mg/kg/day, minimally representing 0.4 times the human dose of both piperacillin and tazobactam based on body-surface area (mg/m2).
A fertility and general reproduction study in rats using intraperitoneal administration of tazobactam or the combination piperacillin/tazobactam prior to mating and through the end of gestation, reported a decrease in litter size in the presence of maternal toxicity at 640 mg/kg/day tazobactam (4 times the human dose of tazobactam based on body-surface area), and decreased litter size and an increase in fetuses with ossification delays and variations of ribs, concurrent with maternal toxicity at ≥640/160 mg/kg/day piperacillin/tazobactam (0.5 times and 1 times the human dose of piperacillin and tazobactam, respectively, based on body-surface area).
Peri/postnatal development in rats was impaired with reduced pup weights, increased stillbirths, and increased pup mortality concurrent with maternal toxicity after intraperitoneal administration of tazobactam alone at doses ≥320 mg/kg/day (2 times the human dose based on body surface area) or of the combination piperacillin/tazobactam at doses ≥640/160 mg/kg/day (0.5 times and 1 times the human dose of piperacillin and tazobactam, respectively, based on body-surface area) from gestation day 17 through lactation day 21.
8.2 Lactation
Risk Summary
Piperacillin is excreted in human milk; tazobactam concentrations in human milk have not been studied. No information is available on the effects of piperacillin and tazobactam on the breastfed child or on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for piperacillin and tazobactam and any potential adverse effects on the breastfed child from piperacillin and tazobactam or from the underlying maternal condition.
8.4 Pediatric Use
Use of piperacillin and tazobactam in pediatric patients 2 months of age or older with appendicitis and/or peritonitis is supported by evidence from well-controlled studies and pharmacokinetic studies in adults and in pediatric patients. This includes a prospective, randomized, comparative, open-label clinical trial with 542 pediatric patients 2 to 12 years of age with complicated intra-abdominal infections, in which 273 pediatric patients received piperacillin/tazobactam. Safety and efficacy in pediatric patients less than 2 months of age have not been established [see Clinical Pharmacology (12) and Dosage and Administration (2)].
It has not been determined how to adjust piperacillin and tazobactam for injection dosage in pediatric patients with renal impairment.
8.5 Geriatric Use
Patients over 65 years are not at an increased risk of developing adverse effects solely because of age. However, dosage should be adjusted in the presence of renal impairment [see Dosage and Administration (2)].
In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Piperacillin and tazobactam for injection contains 54 mg (2.35 mEq) of sodium per gram of piperacillin in the combination product. At the usual recommended doses, patients would receive between 648 and 864 mg/day (28.2 and 37.6 mEq) of sodium. The geriatric population may respond with a blunted natriuresis to salt loading. This may be clinically important with regard to such diseases as congestive heart failure.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
8.6 Renal Impairment
In patients with creatinine clearance ≤ 40 mL/min and dialysis patients (hemodialysis and CAPD), the intravenous dose of piperacillin and tazobactam for injection should be reduced to the degree of renal function impairment [see Dosage and Administration (2)].
8.7 Hepatic Impairment
Dosage adjustment of piperacillin and tazobactam for injection is not warranted in patients with hepatic cirrhosis [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
There have been postmarketing reports of overdose with piperacillin/tazobactam. The majority of those events experienced, including nausea, vomiting, and diarrhea, have also been reported with the usual recommended dosages. Patients may experience neuromuscular excitability or convulsions if higher than recommended doses are given intravenously (particularly in the presence of renal failure) [see Warnings and Precautions (5.4)].
Treatment should be supportive and symptomatic according the patient's clinical presentation. Excessive serum concentrations of either piperacillin or tazobactam may be reduced by hemodialysis. Following a single 3.375 g dose of piperacillin/tazobactam, the percentage of the piperacillin and tazobactam dose removed by hemodialysis was approximately 31% and 39%, respectively [see Clinical Pharmacology (12)].
-
11 DESCRIPTION
Piperacillin and Tazobactam for Injection, USP is an injectable antibacterial combination product consisting of the semisynthetic antibacterial piperacillin sodium and the β-lactamase inhibitor tazobactam sodium for intravenous administration.
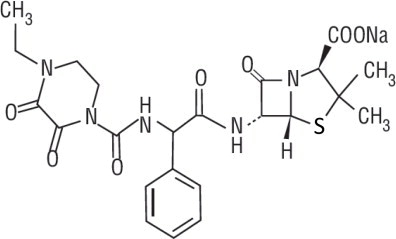
Piperacillin sodium is derived from D(-)-α-aminobenzyl-penicillin. The chemical name of piperacillin sodium is sodium (2S,5R,6R)-6-[(R)-2-(4-ethyl-2,3-dioxo-1-piperazine-carboxamido)-2-phenylacetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate. The chemical formula is C23H26N5NaO7S and the molecular weight is 539.5. The chemical structure of piperacillin sodium is:
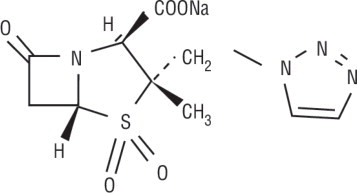
Tazobactam sodium, a derivative of the penicillin nucleus, is a penicillanic acid sulfone. Its chemical name is sodium (2S,3S,5R)-3-methyl-7-oxo-3-(1H-1,2,3-triazol-1-ylmethyl)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate-4, 4-dioxide. The chemical formula is C10H11N4NaO5S and the molecular weight is 322.3. The chemical structure of tazobactam sodium is:
Piperacillin and tazobactam for injection, USP is a white to off-white sterile, cryodesiccated powder consisting of piperacillin and tazobactam as their sodium salts packaged in glass vials.
Each piperacillin and tazobactam for injection, USP 2.25 gram single dose vial contains an amount of drug sufficient for withdrawal of piperacillin sodium equivalent to 2 grams of piperacillin, USP and tazobactam sodium equivalent to 0.25 gram of tazobactam, USP. Each vial contains 4.7 mEq (108 mg) of sodium.
Each piperacillin and tazobactam for injection, USP 3.375 gram single dose vial contains an amount of drug sufficient for withdrawal of piperacillin sodium equivalent to 3 grams of piperacillin, USP and tazobactam sodium equivalent to 0.375 gram of tazobactam, USP. Each vial contains 7.05 mEq (162 mg) of sodium.
Each piperacillin and tazobactam for injection, USP 4.5 gram single dose vial contains an amount of drug sufficient for withdrawal of piperacillin sodium equivalent to 4 grams of piperacillin, USP and tazobactam sodium equivalent to 0.5 gram of tazobactam, USP. Each vial contains 9.4 mEq (216 mg) of sodium.
Piperacillin and tazobactam for injection, USP contains a total of 2.35 mEq (54 mg) of sodium (Na+) per gram of piperacillin in the combination product.
Meets the USP Organic Impurities Test 4.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Piperacillin and tazobactam is an antibacterial drug [see Microbiology (12.4)].
12.2 Pharmacodynamics
The pharmacodynamic parameter for piperacillin/tazobactam that is most predictive of clinical and microbiological efficacy is time above MIC.
12.3 Pharmacokinetics
The mean and coefficients of variation (CV%) for the pharmacokinetic parameters of piperacillin and tazobactam after multiple intravenous doses are summarized in Table 6.
Table 6: Mean (CV%) Piperacillin and Tazobactam PK Parameters a Piperacillin and tazobactam were given in combination, infused over 30 minutes.
b Numbers in parentheses are coefficients of variation (CV%).
Piperacillin Piperacillin/
Tazobactam
DoseaCmax
mcg/mLAUCb
mcgh/mLCL
mL/minV
LT1/2
hCLR mL/min 2.25 g 134 131 (14) 257 17.4 0.79 -- 3.375 g 242 242 (10) 207 15.1 0.84 140 4.5 g 298 322 (16) 210 15.4 0.84 -- Tazobactam Piperacillin/
Tazobactam
DoseaCmax
mcg/mLAUCb
mcgh/mLCL
mL/minV
LT1/2
hCLR mL/min 2.25 g 15 16 (21) 258 17 0.77 -- 3.375 g 24 25 (8) 251 14.8 0.68 166 4.5 g 34 39.8 (15) 206 14.7 0.82 -- Peak plasma concentrations of piperacillin and tazobactam are attained immediately after completion of an intravenous infusion of piperacillin and tazobactam. Piperacillin plasma concentrations, following a 30-minute infusion of piperacillin and tazobactam, were similar to those attained when equivalent doses of piperacillin were administered alone. Steady-state plasma concentrations of piperacillin and tazobactam were similar to those attained after the first dose due to the short half-lives of piperacillin and tazobactam.
Distribution
Both piperacillin and tazobactam are approximately 30% bound to plasma proteins. The protein binding of either piperacillin or tazobactam is unaffected by the presence of the other compound. Protein binding of the tazobactam metabolite is negligible.
Piperacillin and tazobactam are widely distributed into tissues and body fluids including intestinal mucosa, gallbladder, lung, female reproductive tissues (uterus, ovary, and fallopian tube), interstitial fluid, and bile. Mean tissue concentrations are generally 50% to 100% of those in plasma. Distribution of piperacillin and tazobactam into cerebrospinal fluid is low in subjects with non-inflamed meninges, as with other penicillins (see Table 7).
Table 7: Piperacillin/Tazobactam Concentrations in Selected Tissues and Fluids after Single 4 g/0.5 g 30-min IV Infusion of Piperacillin and Tazobactam for Injection a Each subject provided a single sample.
b Time from the start of the infusion
Tissue or
FluidNa Sampling
periodb
(h)Mean PIP
Concentration
Range
(mg/L)Tissue:
Plasma RangeTazo
Concentration
Range
(mg/L)Tazo Tissue:
Plasma RangeSkin 35 0.5 to 4.5 34.8 to 94.2 0.6 to 1.1 4 to 7.7 0.49 to 0.93 Fatty Tissue 37 0.5 to 4.5 4 to 10.1 0.097 to 0.115 0.7 to 1.5 0.1 to 0.13 Muscle 36 0.5 to 4.5 9.4 to 23.3 0.29 to 0.18 1.4 to 2.7 0.18 to 0.3 Proximal
Intestinal
Mucosa7 1.5 to 2.5 31.4 0.55 10.3 1.15 Distal
Intestinal
Mucosa7 1.5 to 2.5 31.2 0.59 14.5 2.1 Appendix 22 0.5 to 2.5 26.5 to 64.1 0.43 to 0.53 9.1 to 18.6 0.8 to 1.35 Metabolism
Piperacillin is metabolized to a minor microbiologically active desethyl metabolite. Tazobactam is metabolized to a single metabolite that lacks pharmacological and antibacterial activities.
Excretion
Following single or multiple piperacillin and tazobactam doses to healthy subjects, the plasma half-life of piperacillin and of tazobactam ranged from 0.7 to 1.2 hours and was unaffected by dose or duration of infusion.
Both piperacillin and tazobactam are eliminated via the kidney by glomerular filtration and tubular secretion. Piperacillin is excreted rapidly as unchanged drug with 68% of the administered dose excreted in the urine. Tazobactam and its metabolite are eliminated primarily by renal excretion with 80% of the administered dose excreted as unchanged drug and the remainder as the single metabolite. Piperacillin, tazobactam and desethyl piperacillin are also secreted into the bile.
Specific Populations
Renal Impairment
After the administration of single doses of piperacillin/tazobactam to subjects with renal impairment, the half-life of piperacillin and of tazobactam increases with decreasing creatinine clearance. At creatinine clearance below 20 mL/min, the increase in half-life is twofold for piperacillin and fourfold for tazobactam compared to subjects with normal renal function. Dosage adjustments for piperacillin and tazobactam are recommended when creatinine clearance is below 40 mL/min in patients receiving the usual recommended daily dose of piperacillin and tazobactam for injection. See Dosage and Administration (2) for specific recommendations for the treatment of patients with renal-impairment.
Hemodialysis removes 30% to 40% of a piperacillin/tazobactam dose with an additional 5% of the tazobactam dose removed as the tazobactam metabolite. Peritoneal dialysis removes approximately 6% and 21% of the piperacillin and tazobactam doses, respectively, with up to 16% of the tazobactam dose removed as the tazobactam metabolite. For dosage recommendations for patients undergoing hemodialysis [see Dosage and Administration (2)].
Hepatic Impairment
The half-life of piperacillin and of tazobactam increases by approximately 25% and 18%, respectively, in patients with hepatic cirrhosis compared to healthy subjects. However, this difference does not warrant dosage adjustment of piperacillin and tazobactam due to hepatic cirrhosis.
Pediatrics
Piperacillin and tazobactam pharmacokinetics were studied in pediatric patients 2 months of age and older. The clearance of both compounds is slower in the younger patients compared to older children and adults.
In a population PK analysis, estimated clearance for 9 month-old to 12 year-old patients was comparable to adults, with a population mean (SE) value of 5.64 (0.34) mL/min/kg. The piperacillin clearance estimate is 80% of this value for pediatric patients 2 to 9 months old. In patients younger than 2 months of age, clearance of piperacillin is slower compared to older children; however, it is not adequately characterized for dosing recommendations. The population mean (SE) for piperacillin distribution volume is 0.243 (0.011) L/kg and is independent of age.
Geriatrics
The impact of age on the pharmacokinetics of piperacillin and tazobactam was evaluated in healthy male subjects, aged 18 to 35 years (n=6) and aged 65 to 80 years (n=12). Mean half-life for piperacillin and tazobactam was 32% and 55% higher, respectively, in the elderly compared to the younger subjects. This difference may be due to age-related changes in creatinine clearance.
Drug Interactions
The potential for pharmacokinetic drug interactions between piperacillin and tazobactam and aminoglycosides, probenecid, vancomycin, heparin, vecuronium, and methotrexate has been evaluated [see Drug Interactions (7)].
12.4 Microbiology
Mechanism of Action
Piperacillin sodium exerts bactericidal activity by inhibiting septum formation and cell wall synthesis of susceptible bacteria. In vitro, piperacillin is active against a variety of Gram-positive and Gram-negative aerobic and anaerobic bacteria. Tazobactam sodium has little clinically relevant in vitro activity against bacteria due to its reduced affinity to penicillin-binding proteins. It is, however, a β-lactamase inhibitor of the Molecular class A enzymes, including Richmond-Sykes class III (Bush class 2b & 2b') penicillinases and cephalosporinases. It varies in its ability to inhibit class II and IV (2a & 4) penicillinases. Tazobactam does not induce chromosomally-mediated β-lactamases at tazobactam concentrations achieved with the recommended dosage regimen.
Spectrum of Activity
Piperacillin/tazobactam has been shown to be active against most isolates of the following microorganisms both in vitro and in clinical infections [see Indications and Usage (1)].
Gram-positive bacteria:
Staphylococcus aureus (methicillin susceptible isolates only)
Gram-negative bacteria:
Acinetobacter baumannii
Escherichia coli
Haemophilus influenzae (excluding β-lactamase negative, ampicillin-resistant isolates)
Klebsiella pneumoniae
Pseudomonas aeruginosa (given in combination with an aminoglycoside to which the isolate is susceptible)Anaerobic bacteria:
Bacteroides fragilis group (B. fragilis, B. ovatus, B. thetaiotaomicron, and B. vulgatus)The following in vitro data are available, but their clinical significance is unknown.
At least 90% of the following microorganisms exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for piperacillin/tazobactam. However, the safety and effectiveness of piperacillin/tazobactam in treating clinical infections due to these bacteria have not been established in adequate and well-controlled clinical trials.
Gram-positive bacteria:
Enterococcus faecalis (ampicillin or penicillin-susceptible isolates only)
Staphylococcus epidermidis (methicillin susceptible isolates only)
Streptococcus agalactiae†
Streptococcus pneumoniae† (penicillin-susceptible isolates only)
Streptococcus pyogenes†
Viridans group streptococci†Gram-negative bacteria:
Citrobacter koseri
Moraxella catarrhalis
Morganella morganii
Neisseria gonorrhoeae
Proteus mirabilis
Proteus vulgaris
Serratia marcescens
Providencia stuartii
Providencia rettgeri
Salmonella entericaAnaerobic bacteria:
Clostridium perfringens
Bacteroides distasonis
Prevotella melaninogenica† These are not β-lactamase producing bacteria and, therefore, are susceptible to piperacillin alone.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenicity studies in animals have not been conducted with piperacillin/tazobactam, piperacillin, or tazobactam.
Piperacillin/Tazobactam
Piperacillin/tazobactam was negative in microbial mutagenicity assays, the unscheduled DNA synthesis (UDS) test, a mammalian point mutation (Chinese hamster ovary cell HPRT) assay, and a mammalian cell (BALB/c-3T3) transformation assay. In vivo, piperacillin/tazobactam did not induce chromosomal aberrations in rats.
Piperacillin/tazobactam
Reproduction studies have been performed in rats and have revealed no evidence of impaired fertility when piperacillin/tazobactam is administered intravenously up to a dose of 1280/320 mg/kg piperacillin/tazobactam, which is similar to the maximum recommended human daily dose based on body-surface area (mg/m2).
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Piperacillin and Tazobactam for Injection, USP is supplied in single-dose vials as follows:
NDC Piperacillin and Tazobactam for Injection, USP Package Factor 25021-164-30 Each 2.25 gram vial provides piperacillin sodium equivalent to 2 grams of piperacillin, 10 vials per carton USP and tazobactam sodium equivalent to 0.25 gram of tazobactam, USP. Each vial contains 4.7 mEq (108 mg) of sodium. 25021-165-30 Each 3.375 gram vial provides piperacillin sodium equivalent to 3 grams of piperacillin, 10 vials per carton USP and tazobactam sodium equivalent to 0.375 gram of tazobactam, USP. Each vial contains 7.05 mEq (162 mg) of sodium. 25021-166-48 Each 4.5 gram vial provides piperacillin sodium equivalent to 4 grams of piperacillin, 10 vials per carton USP and tazobactam sodium equivalent to 0.5 gram of tazobactam, USP. Each vial contains 9.4 mEq (216 mg) of sodium. -
17 PATIENT COUNSELING INFORMATION
Serious Hypersensitivity Reactions
Advise patients, their families, or caregivers that serious hypersensitivity reactions, including serious allergic cutaneous reactions, could occur that require immediate treatment. Ask them about any previous hypersensitivity reactions to piperacillin and tazobactam, other beta-lactams (including cephalosporins), or other allergens [see Warnings and Precautions (5.2)].
Diarrhea
Advise patients, their families, or caregivers that diarrhea is a common problem caused by antibacterial drugs which usually ends when the drug is discontinued. Sometimes after starting treatment with antibacterial drugs, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the drug. If this occurs, patients should contact their physician as soon as possible.
Antibacterial Resistance
Counsel patients that antibacterial drugs including piperacillin and tazobactam for injection should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When piperacillin and tazobactam for injection is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by piperacillin and tazobactam for injection or other antibacterial drugs in the future.
Counsel patients that piperacillin and tazobactam can cross the placenta in humans and is excreted in human milk.
Brands listed are the trademarks of their respective owners.
SAGENT®
Mfd. for SAGENT Pharmaceuticals
Schaumburg, IL 60195 (USA)
Made in India
©2018 Sagent Pharmaceuticals, Inc.Revised: December 2018
SAGENT Pharmaceuticals®
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Vial Label
NDC: 25021-164-30
Rx only
Piperacillin and Tazobactam for Injection, USP
2.25 grams per vial
For Intravenous Use Only
Single-Dose Vial
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Vial Label
NDC: 25021-165-30
Rx only
Piperacillin and Tazobactam for Injection, USP
3.375 grams per vial
For Intravenous Use Only
Single-Dose Vial
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Vial Label
NDC: 25021-166-48
Rx only
Piperacillin and Tazobactam for Injection, USP
4.5 grams per vial
For Intravenous Use Only
Single-Dose Vial
-
INGREDIENTS AND APPEARANCE
PIPERACILLIN, TAZOBACTAM
piperacillin sodium, tazobactam sodium injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 25021-164 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Piperacillin Sodium (UNII: M98T69Q7HP) (Piperacillin Anhydrous - UNII:9I628532GX) Piperacillin Anhydrous 2 g in 10 mL Tazobactam Sodium (UNII: UXA545ABTT) (Tazobactam - UNII:SE10G96M8W) Tazobactam 0.25 g in 10 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 25021-164-30 10 in 1 CARTON 06/16/2011 1 10 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065498 06/16/2011 PIPERACILLIN, TAZOBACTAM
piperacillin sodium, tazobactam sodium injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 25021-165 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Piperacillin Sodium (UNII: M98T69Q7HP) (Piperacillin Anhydrous - UNII:9I628532GX) Piperacillin Anhydrous 3 g in 15 mL Tazobactam Sodium (UNII: UXA545ABTT) (Tazobactam - UNII:SE10G96M8W) Tazobactam 0.375 g in 15 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 25021-165-30 10 in 1 CARTON 06/16/2011 1 15 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065498 06/16/2011 PIPERACILLIN, TAZOBACTAM
piperacillin sodium, tazobactam sodium injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 25021-166 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Piperacillin Sodium (UNII: M98T69Q7HP) (Piperacillin Anhydrous - UNII:9I628532GX) Piperacillin Anhydrous 4 g in 20 mL Tazobactam Sodium (UNII: UXA545ABTT) (Tazobactam - UNII:SE10G96M8W) Tazobactam 0.5 g in 20 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 25021-166-48 10 in 1 CARTON 06/16/2011 1 20 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065498 06/16/2011 Labeler - Sagent Pharmaceuticals (796852890)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.