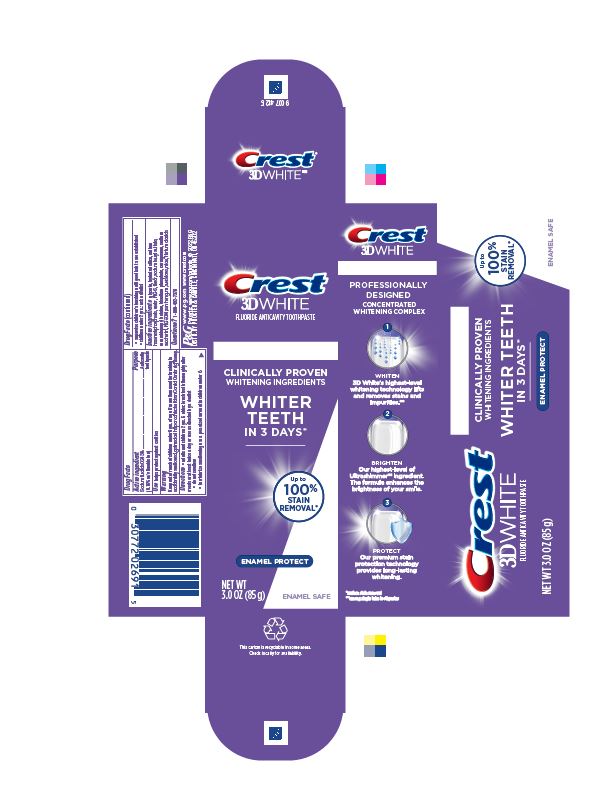
Crest 3D White Enamel Protect Toothpaste
Crest 3D White by
Drug Labeling and Warnings
Crest 3D White by is a Otc medication manufactured, distributed, or labeled by The Procter & Gamble Manufacturing Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CREST 3D WHITE ENAMEL PROTECT- sodium fluoride paste, dentifrice
The Procter & Gamble Manufacturing Company
----------
Crest 3D White Enamel Protect Toothpaste
Directions
- adults and children 2 yrs. & older: brush teeth thoroughly after meals or at least twice a day or use as directed by a dentist
- do not swallow
- to minimize swallowing use a pea-sized amount in children under 6
- supervise children's brushing until good habits are established
- children under 2 yrs.: ask a dentist
| CREST 3D WHITE
ENAMEL PROTECT
sodium fluoride paste, dentifrice |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - The Procter & Gamble Manufacturing Company (004238200) |
Revised: 1/2026
Document Id: 47e55daf-589f-e8d2-e063-6294a90aeb53
Set id: d4ec0f3e-5c33-0229-e053-2995a90a57b0
Version: 8
Effective Time: 20260108
The Pr
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.