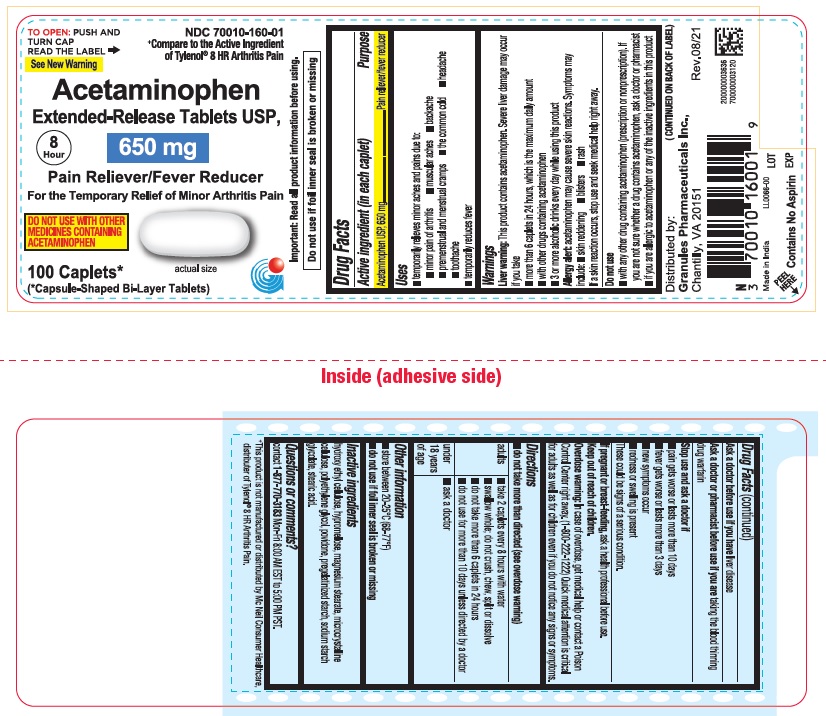
ACETAMINOPHEN tablet, extended release
ACETAMINOPHEN by
Drug Labeling and Warnings
ACETAMINOPHEN by is a Otc medication manufactured, distributed, or labeled by Granules Pharmaceuticals Inc., Granules India Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT (IN EACH CAPLET)
- PURPOSE
-
USES
For Arthritis Pain label
temporarily relieves minor aches and pains due to:
minor pain of arthritis
muscular aches
backache
premenstrual and menstrual cramps
the common cold
headache
toothache
temporarily reduces fever
For Muscle Aches & Pain label
temporarily relieves minor aches and pains due to:
muscular aches
backache
minor pain of arthritis
toothache
premenstrual and menstrual cramps
headache
the common cold
temporarily reduces fever
-
WARNINGS
Liver warning: This product contains acetaminophen. Severe Liver damage may occur if you take
more than 6 caplets in 24 hours, which is the maximum daily amount
with other drugs containing acetaminophen
3 or more alcoholic drinks everyday while using this productAllergy alert: acetaminophen may cause severe skin reactions
Symptoms may include:
skin reddening
blisters
rash
If a skin reaction occurs, stop use and seek medical help right away - Do not use
- Ask a doctor before use if you have
- Ask a doctor or pharmacist before use if you are
- Stop use and ask doctor if
- If pregnant or breast-feeding
- Keep out of reach of children
-
DIRECTIONS
For Arthritis Pain Label
do not take more than directed (see overdose warning)
adults take 2 caplets every 8 hours with water
swallow whole; do not crush, chew, split or dissolve
do not take more than 6 caplets in 24 hours
do not use for more than 10 days unless directed by a doctorunder 18 years
of ageask a doctor
For Muscle Ache and Pain label
do not take more than directed (see overdose warning)
adults and children
12 years of age and overtake 2 caplets every 8 hours with water
swallow whole; do not crush, chew, split or dissolve
do not take more than 6 caplets in 24 hours
do not use for more than 10 days unless directed by a doctorchildren under
12 yearsdo not use - OTHER INFORMATION
- INACTIVE INGREDIENTS
- QUESTIONS OR COMMENTS ?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN
acetaminophen tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 70010-160 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 650 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE K30 (UNII: U725QWY32X) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) HYDROXYETHYL CELLULOSE (140 CPS AT 5%) (UNII: 8136Y38GY5) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) Product Characteristics Color white Score no score Shape CAPSULE Size 19mm Flavor Imprint Code G650 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70010-160-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 02/15/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211544 02/15/2022 Labeler - Granules Pharmaceuticals Inc. (079825711) Registrant - Granules India Limited (915000087)
Trademark Results [ACETAMINOPHEN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ACETAMINOPHEN 85615223 not registered Dead/Abandoned |
General Merchandise importers and Expoters 2012-05-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.