CINRYZE- human c1-esterase inhibitor injection, powder, lyophilized, for solution
Cinryze by
Drug Labeling and Warnings
Cinryze by is a Other medication manufactured, distributed, or labeled by ViroPharma Biologics LLC, Sanquin Plasma Products (SPP), Plasma Industries Belgium SCRL, Baxter Aktiengesellschaft, Siegfried Hameln GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CINRYZE® safely and effectively. See full prescribing information for CINRYZE.
CINRYZE (C1 Esterase Inhibitor [Human])
For Intravenous Use, Freeze-Dried Powder for Reconstitution
Initial U.S. Approval: 2008.INDICATIONS AND USAGE
CINRYZE is a C1 esterase inhibitor indicated for routine prophylaxis against angioedema attacks in adults, adolescents and pediatric patients (6 years of age and older) with Hereditary Angioedema (HAE). (1)
DOSAGE AND ADMINISTRATION
For Intravenous Use Only
- Prior to reconstitution, protect from light. (2.2)
- A silicone-free syringe is recommended. (2.2)
- Store at 36°F to 77°F (2°C to 25°C). Do not freeze. (16)
- To obtain a 500 U CINRYZE dose, reconstitute one CINRYZE vial with one vial of Sterile Water for Injection, USP (5 mL). To obtain a 1,000 U CINRYZE dose, reconstitute two CINRYZE vials with two vials of Sterile Water for Injection, USP (5 mL each). Use aseptic sterile technique. (2.2)
- Administer at room temperature within 3 hours of reconstitution. (2.2)
Routine Prophylaxis Dosing (2.1)
Adults and adolescents (12 years old and above)
Indication Dose Infusion rate - * Doses up to 2,500 U (not exceeding 100 U/kg) every 3 or 4 days may be considered based on individual patient response.
Routine prophylaxis against HAE attacks 1,000 Units Intravenous every 3 or 4 days* 1 mL/min
(10 min)Children (6 to 11 years old)
Indication Dose Infusion rate - * Doses up to 1,000 U every 3 to 4 days may be considered based on individual patient response.
Routine prophylaxis against HAE attacks 500 Units Intravenous every 3 or 4 days* 1 mL/min
(5 min)DOSAGE FORMS AND STRENGTHS
Approximately 500 Units (lyophilized) in an 8 mL vial. (3)
CONTRAINDICATIONS
Patients who have manifested life-threatening immediate hypersensitivity reactions, including anaphylaxis, to the product (4).
WARNINGS AND PRECAUTIONS
- Hypersensitivity reactions may occur. Have epinephrine immediately available for treatment of acute severe hypersensitivity reaction (5.1)
- Serious arterial and venous thromboembolic (TE) events have been reported at the recommended dose of C1 Esterase Inhibitor (Human) products, including CINRYZE, following administration in patients with HAE. Risk factors may include presence of an indwelling venous catheter/access device, prior history of thrombosis, underlying atherosclerosis, use of oral contraceptives, certain androgens, morbid obesity, and immobility. Benefits of CINRYZE for routine prophylaxis of HAE attacks should be weighed against the risks of TE events in patients with underlying risk factors. Monitor patients with known risk factors for TE events during and after CINRYZE administration. TE events have been reported following administration of a C1 Esterase Inhibitor (Human) product when used off-label at higher than labeled doses. (5.2)
- CINRYZE is made from human plasma and may contain infectious agents e.g. viruses and, theoretically, the Creutzfeldt-Jakob disease agent. (5.3)
ADVERSE REACTIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose
2.2 Preparation
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Thromboembolic Events
5.3 Transmissible Infectious Agents
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
6.3 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
For intravenous use only.
2.1 Dose
Table 1 Routine Prophylaxis Dosing in Adults, Adolescents, and Pediatric Patients. Patient Population Indication Dose Infusion Rate Adults and adolescents
(12 years old and above)Routine prophylaxis against HAE attacks 1,000 Units (U) Intravenous (IV) every 3 or 4 days. For patients who have not responded adequately to 1,000 U of CINRYZE every 3 or 4 days, doses up to 2,500 U (not to exceed 100 U/kg) every 3 or 4 days may be considered based on individual patient response. 1 mL/min
(10 min)Pediatric patients
(6 to 11 years old)Routine prophylaxis against HAE attacks 500 U, IV every 3 or 4 days. The dose may be adjusted according to individual response, up to 1,000 U every 3 to 4 days. 1 mL/min
(5 min)2.2 Preparation
- Protect CINRYZE from light prior to reconstitution.
- A silicone-free syringe is recommended for reconstitution and administration of CINRYZE.
- Inspect the reconstituted product for particulate matter prior to administration; do not use if particles are observed or if solution is turbid. The reconstituted solution is colorless to slightly blue.
- Each vial of CINRYZE is for single use only. Promptly use any vial that has been entered and discard partially used vials in accordance with biohazard procedures. CINRYZE contains no preservative.
- Do not mix CINRYZE with other materials.
- Do not use if frozen.
- Do not use after expiration date.
- Use aseptic technique during the reconstitution procedure.
- Bring the CINRYZE (powder) and Sterile Water for Injection, USP (diluent) (not supplied) to room temperature if refrigerated.
- Remove caps from the CINRYZE and diluent vials.
- Cleanse stoppers with an alcohol wipe or swab, and allow them to dry prior to use.
- Remove protective covering from the top of the Mix2Vial transfer device package. Do not remove the device from the package.
- Note: Diluent vial must be accessed prior to the vial of CINRYZE to prevent loss of vacuum.
Reconstitution
One vial of reconstituted CINRYZE is used for a single 500 U dose. Two vials of reconstituted CINRYZE are combined for a single 1,000 U dose. Sterile Water for Injection, USP, is required and not supplied with CINRYZE.
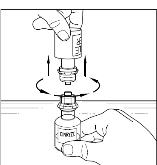
- Place diluent on a flat surface and insert the blue end of the device into the diluent vial, pushing down until the spike penetrates through the center of the diluent vial stopper and the device snaps in place (Figure 1). The Mix2Vial transfer device must be positioned completely vertical prior to penetrating the stopper closure.
Figure 1
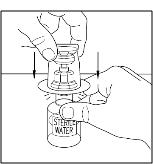
- Remove the plastic package and discard it (Figure 2). Take care not to touch the exposed end of the device.
Figure 2
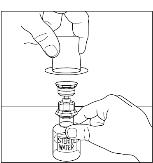
- Place vial of CINRYZE on a flat surface. Invert diluent vial containing 5 mL of Sterile Water for Injection, USP, and insert the clear end into the CINRYZE vial, pushing down until the spike penetrates the rubber stopper and the device snaps into place. The Mix2Vial transfer device must be positioned completely vertical prior to penetrating the stopper closure. The Sterile Water for Injection, USP will automatically flow into the vial of CINRYZE (Figure 3), because the vacuum in the vial will draw in the diluent. If there is no vacuum in the vial, do not use the product.
Figure 3
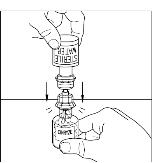
- Gently swirl (do not shake) the CINRYZE vial until all powder is dissolved. Be sure that CINRYZE is completely dissolved (Figure 4).
Figure 4
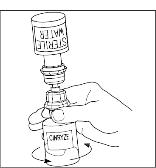
- Disconnect the Sterile Water for Injection, USP vial by turning it counterclockwise (Figure 5). Do not remove the clear end of the Mix2Vial transfer device from the vial of CINRYZE.
Figure 5
One vial of reconstituted CINRYZE contains 5 mL of C1 esterase inhibitor at a concentration of 100 U/mL. Reconstitute one vial of CINRYZE for one 500 U dose. Reconstitute two vials of CINRYZE for one 1,000 U dose. Repeat steps 1 to 5 above using an additional package containing a Mix2Vial transfer device to reconstitute the second of two vials of CINRYZE. Do not reuse the Mix2Vial transfer device. CINRYZE must be administered at room temperature within 3 hours after reconstitution. For higher doses up to 2,500 U (not exceeding 100 U/kg) additional vial(s) will need to be reconstituted.
2.3 Administration
The procedures below are provided as general guidelines for the reconstitution and administration of CINRYZE. Use either the Mix2Vial® transfer device or a commercially available double-ended needle.
Always work on a clean surface and wash your hands before performing the following procedures.
Reconstitution, product administration, and handling of the administration set and needles must be done with caution. Percutaneous puncture with a needle contaminated with blood can transmit infectious viruses including HIV (AIDS) and hepatitis. Obtain immediate medical attention if injury occurs. Place needles in a sharps container after single use. Discard all equipment, including any reconstituted CINRYZE, in an appropriate container.
One vial of reconstituted CINRYZE is used for a single 500 U dose.
Two vials of reconstituted CINRYZE are combined for a single 1,000 U dose.
For higher doses of up to 2,500 U, combine the content of the relevant number of vials.
Instructions for Use
- Use aseptic technique.
- After reconstitution, the solution should be clear with no evidence of turbidity. Reconstituted solution should be colorless to slightly blue. Do not use if solution is turbid or otherwise discolored.
- Please refer to the illustrations in steps 7 to 9 included within the Patient Information Leaflet. Utilizing a sterile, disposable 10 mL syringe, draw back the plunger to admit 5 mL air into the syringe.
- Attach the syringe onto the top of the clear end of the Mix2Vial transfer device by turning it clockwise.
- Invert the vial and inject air into the solution and then slowly withdraw the reconstituted CINRYZE into the syringe.
- Detach the syringe from the vial by turning it counterclockwise and releasing it from the clear end of the Mix2Vial transfer device.
- For a 1,000 U dose, using the same syringe, repeat steps 3 to 6 with a second vial of CINRYZE to make the complete dose.
- Promptly administer CINRYZE after preparation in the syringe and do not use if particles are observed or if the solution is turbid.
- Attach a suitable needle or infusion set with winged adapter, and inject intravenously. As a guideline, administer CINRYZE by intravenous injection at a rate of 1 mL per minute. A single dose of 500 U (reconstituted in 5 mL) of CINRYZE should be administered over 5 minutes. A single dose of 1,000 U (reconstituted in 10 mL) of CINRYZE should be administered over 10 minutes. (see Dosage and Administration [2].) Please refer to the illustration in step 3 of the self-administration section within the Patient Information Leaflet.
- Dispose of all unused solution, the empty vial(s), and the used needles and syringes in an appropriate container for throwing away waste that might hurt others if not handled properly.
-
3 DOSAGE FORMS AND STRENGTHS
- CINRYZE (Freeze-Dried powder for Reconstitution) is a lyophilized preparation available in a single-use vial that contains 500 U human C1 esterase inhibitor.
- Each vial must be reconstituted with 5 mL of Sterile Water for Injection, USP (diluent) (not supplied).
- One reconstituted vial must be used to make a single, 500 U, dose. Two reconstituted vials must be used to make a single, 1,000 U dose.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Severe hypersensitivity reactions may occur. The signs and symptoms of hypersensitivity reactions may include the appearance of hives, urticaria, tightness of the chest, wheezing, hypotension and/or anaphylaxis experienced during or after injection of CINRYZE.
Consider treatment methods carefully, because hypersensitivity reactions may have symptoms similar to HAE attacks.
In case of hypersensitivity, discontinue CINRYZE infusion and institute appropriate treatment. Have epinephrine immediately available for treatment of acute severe hypersensitivity reaction. (see Patient Counseling Information [17])
5.2 Thromboembolic Events
Serious arterial and venous thromboembolic (TE) events have been reported at the recommended dose of C1 Esterase Inhibitor (Human) products, including CINRYZE, following administration in patients with HAE. Risk factors may include presence of an indwelling venous catheter/access device, prior history of thrombosis, underlying atherosclerosis, use of oral contraceptives, certain androgens, morbid obesity, and immobility. Benefits of CINRYZE for routine prophylaxis of HAE attacks should be weighed against the risks of TE events in patients with underlying risk factors. Monitor patients with known risk factors for TE events during and after CINRYZE administration.
TE events have been reported following administration of a C1 Esterase Inhibitor (Human) product when used off-label at higher than labeled doses1.
In an open-label trial further investigating the use of CINRYZE for prevention (n=146) of HAE attacks, 5 serious TE events (including myocardial infarction, deep vein thrombosis, pulmonary embolism and 2 events of cerebrovascular accident) occurred. Subjects had underlying risk factors for TE events.
In the post-approval open-label study (n=20) there were no systemic TE events in subjects who received CINRYZE up to 2,500 U (not exceeding 100 U/kg) every 3 or 4 days for up to 12 months. One subject developed a blood clot in an intravenous central catheter, which was treated without systemic complication. (see Adverse Reactions [6.1]).
5.3 Transmissible Infectious Agents
Because CINRYZE is made from human blood, it may carry a risk of transmitting infectious agents, e.g. viruses, and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent (see Description [11]). ALL infections thought by a physician possibly to have been transmitted by CINRYZE should be reported by the physician or other healthcare provider to Shire Medical Information. [1-800-828-2088]. The physician should discuss the risks and benefits of this product with the patient, before prescribing or administering it to the patient. (see Patient Counseling Information [17])
-
6 ADVERSE REACTIONS
The most common adverse reactions (≥5%) observed were headache, nausea, rash, vomiting, and fever.
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Twenty-four subjects age 9 to 73 years old were evaluated in the randomized, placebo-controlled, crossover, routine prophylaxis trial. There were no serious adverse reactions in the randomized, placebo-controlled, crossover, routine prophylaxis trial.
Adverse reactions in the randomized, placebo-controlled, crossover, routine prophylaxis trial (n=24) that occurred in at least two subjects (≥8%) receiving CINRYZE are given in Table 2:
Table 2 Adverse Reactions in the Randomized, Placebo-Controlled, Crossover, Routine Prophylaxis Trial. Adverse Reaction Number of Adverse Reactions Number of Subjects (N = 24) Rash 8 5 Headache 4 4 Pruritus 2 2 Vomiting 2 2 In an open-label follow-on trial, 146 subjects age 3 to 82 years old received a median of 243.5 days of CINRYZE (maximum = 959 days). The most common adverse reaction observed was headache. No subjects were discontinued due to an adverse reaction.
Adverse reactions in the open-label follow-on trial (n=146) that occurred in at least three subjects (≥2%) receiving CINRYZE, are given in Table 3:
Table 3 Adverse Reactions in the Open-Label Follow-On Trial. Adverse Reaction Number (%) of Subjects (N=146) with Adverse Reaction Number (%) of Infusion Days (N=11,435) with Adverse Reaction Headache 28 (19) 62 (0.5) Nausea 26 (18) 29 (0.3) Rash 15 (10) 30 (0.3) Vomiting 15 (10) 17 (0.1) Pyrexia (fever) 7 (5) 7 (<0.1) Catheter Site Pain 4 (3) 5 (<0.1) Dizziness 3 (2) 4 (<0.1) Erythema 3 (2) 3 (<0.1) Pruritus 3 (2) 4 (<0.1) Serious adverse reactions included cerebrovascular accident (see Warnings and Precautions [5.2]).
Twelve pediatric subjects age 7 to 11 years old were evaluated in a randomized, dose-ranging, crossover routine prophylaxis trial (500 U and 1000 U). No new adverse reactions (compared to the placebo-controlled routine prophylaxis trial or the open label follow-on trial) were identified. The adverse reactions among the pediatric subjects were headache, nausea, pyrexia (fever), and infusion site erythema. None of these adverse reactions were severe, and none led to discontinuation of CINRYZE. The safety profile with 500 U or 1000 U of CINRYZE treatment was comparable. Overall, the safety and tolerability of CINRYZE are similar in pediatric, adolescent and adult subjects.
More than 14,000 doses of CINRYZE have been administered to over 260 different subjects in all completed, controlled and open-label clinical studies. All subjects who were evaluated were found negative for seroconversion to parvovirus B19, Hepatitis B, Hepatitis C and HIV. (see Warnings and Precautions [5.3])
A post-approval open-label study assessed escalating doses of CINRYZE (1,500 U, 2,000 U, 2,500 U every 3 or 4 days) as prophylactic therapy in 20 subjects who had an inadequate response (> 1.0 HAE attack/month, regardless of severity) to 1,000 U every 3 or 4 days. The safety profile of doses up to 2,500 U was consistent with previous clinical trial experience at lower doses.
6.2 Immunogenicity
Because CINRYZE is a therapeutic protein, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of anti-C1 esterase inhibitor antibody positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibody development across products cannot be made.
6.3 Postmarketing Experience
Because post marketing reporting of adverse reactions is voluntary and from a population of uncertain size, it is not always possible to reliably estimate the frequency of these reactions or establish a causal relationship to product exposure.
Postmarketing adverse reactions included local infusion site reactions (including inflammation or hematoma at the infusion site) and hypersensitivity.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data with CINRYZE use in pregnant women to inform a drug associated risk. Animal reproduction studies have not been conducted with CINRYZE. It is unknown whether CINRYZE can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. CINRYZE should be given to a pregnant woman only if clearly needed.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data regarding the presence of CINRYZE in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for CINRYZE and any potential adverse effects on the breastfed child from CINRYZE or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of CINRYZE have been evaluated in 12 pediatric subjects with HAE (age range 7 to 11 years old) in a pediatric randomized, dose-ranging, crossover routine prophylaxis trial. (see Clinical Studies [14]). Additionally, four of the 24 subjects in the pediatric/adult randomized, placebo-controlled, crossover, routine prophylaxis trial, were under the age of 18 years (9, 14, 16, and 17 years of age). Overall the safety and tolerability of CINRYZE are similar in pediatric, adolescent and adult subjects. The pharmacokinetics of CINRYZE was evaluated in pediatric subjects (7 to 11 years old). (see Clinical Pharmacology [12]).
8.5 Geriatric Use
Clinical studies of CINRYZE did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
- 10 OVERDOSAGE
-
11 DESCRIPTION
CINRYZE (C1 esterase inhibitor [human]) (Freeze-Dried Powder for Reconstitution) is a sterile, stable, lyophilized preparation of C1 esterase inhibitor derived from human plasma. CINRYZE is manufactured from human plasma purified by a combination of filtration and chromatographic procedures. The specific activity of CINRYZE is 4.0 to 9.0 U/mg protein. The purity is ≥ 90% human C1 esterase inhibitor. Following reconstitution with 5 mL of Sterile Water for Injection, USP, each vial contains approximately 500 U of functionally active C1 esterase inhibitor, pH 6.6 to 7.4, and an osmolality between 200 to 400 mosmol/kg. One Unit (U) of CINRYZE corresponds to the mean quantity of C1 esterase inhibitor present in 1 mL of normal fresh plasma.
CINRYZE, when reconstituted with 5 mL of Sterile Water for Injection, USP contains the following excipients: 4.1 mg/mL sodium chloride, 21 mg/mL sucrose, 2.6 mg/mL trisodium citrate, 2.0 mg/mL L-Valine, 1.2 mg/mL L-Alanine, and 4.5 mg/mL L-Threonine.
The following manufacturing steps are designed to reduce the risk of viral transmission:
- Screening donors at U.S. licensed blood collection centers to rule out infection with Human Immunodeficiency Virus (HIV-1/HIV-2), Hepatitis B Virus, or Hepatitis C Virus.
- Testing plasma pools by in-process NAT for parvovirus B19 via minipool testing and the limit of B19 in the manufacturing pool is set not to exceed 104 IU of B19 DNA per mL.
- Use of two independent viral reduction steps in the manufacture of CINRYZE: pasteurization (heat treatment at 60°C for 10 hours in solution with stabilizers) and nano filtration through two sequential 15 nm filters.
These viral reduction steps, along with a step in the manufacturing process, PEG precipitation, have been validated in a series of in vitro experiments for their capacity to inactivate/remove a wide range of viruses of diverse physicochemical characteristics including: Human Immunodeficiency Virus (HIV), Hepatitis A Virus (HAV), and the following model viruses: Bovine Viral Diarrhea Virus (BVDV) as a model virus for HCV, Canine Parvovirus (CPV) as a model virus for Parvovirus B19, Pseudorabies Virus (PRV) as a model virus for large enveloped DNA viruses (e.g. herpes virus). Total mean log10 reductions are shown in Table 4.
Table 4 Log10 Virus Reduction Factor for Selected Viruses. Process Step Enveloped Viruses - HIV Enveloped Viruses - BVDV Enveloped Viruses - PRV Non-enveloped Viruses - HAV Non-enveloped Viruses - CPV PEG precipitation 5.1 ± 0.2 4.5 ± 0.3 6.0 ± 0.3 2.8 ± 0.2 4.2 ± 0.2 Pasteurization > 6.1 ± 0.2 > 6.7 ± 0.3 > 6.7 ± 0.2 2.8 ± 0.3 0.1 ± 0.3 Nano filtration > 5.6 ± 0.2 > 5.5 ± 0.2 > 6.4 ± 0.3 > 4.9 ± 0.2 > 4.5 ± 0.3 Total reduction > 16.8 > 16.7 > 19.1 > 10.5 > 8.7 -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
C1 inhibitor is a normal constituent of human blood and is one of the serine proteinase inhibitors (serpins). The primary function of C1 inhibitor is to regulate the activation of the complement and intrinsic coagulation (contact system) pathway. C1 inhibitor also regulates the fibrinolytic system. Regulation of these systems is performed through the formation of complexes between the proteinases and the inhibitor, resulting in inactivation of both and consumption of the C1 inhibitor.
HAE patients have low levels of endogenous or functional C1 inhibitor. Although the events that induce attacks of angioedema in HAE patients are not well defined, it is thought by some that increased vascular permeability and the clinical manifestation of HAE attacks are primarily mediated through contact system activation. Suppression of contact system activation by C1 inhibitor through the inactivation of plasma kallikrein and factor XIIa is thought to modulate this vascular permeability by preventing the generation of bradykinin2. Administration of CINRYZE increases plasma levels of C1 inhibitor activity.
12.2 Pharmacodynamics
In clinical studies, the intravenous administration of CINRYZE demonstrated an increase in plasma levels of C1 inhibitor within approximately one hour or less of administration.
Biological activity of CINRYZE was shown in 35 subjects by the subsequent increase in plasma C4 levels from an average of C4 8.1 mg/dL at baseline to C4 8.6 mg/dL 12 hours after infusion of CINRYZE. Pharmacodynamics was investigated in children 7 to 11 years old following IV administration of two dose levels of CINRYZE (500 U and 1,000 U) every 3 or 4 days for 12 weeks to prevent angioedema attacks. C4 levels increased from an average of 6.80 mg/dL at baseline to 7.92 mg/dL for the 500 U dose, and to 9.27 mg/dL for the 1,000 U dose, measured one hour after the final administered dose of the 12-week treatment period.
12.3 Pharmacokinetics
A randomized, parallel group, open-label pharmacokinetics (PK) study of CINRYZE was performed in subjects with non-symptomatic hereditary angioedema (HAE). The subjects received either a single dose of 1,000 U or 1,000 U followed by a second 1,000 U 60 minutes later. The PK results for functional C1 inhibitor are presented in the Table 5.
Table 5 Mean Pharmacokinetic Parameters of Functional C1 Inhibitor. Parameters Single Dose* Double Dose† - * Single dose = 1,000 Units
- † Double dose = 1,000 Units followed by a second 1,000 Units 60 minutes later
- ‡ One Unit is equal to the mean C1 inhibitor concentration of 1 mL of normal human plasma
- § Numbers in parentheses are number of subjects evaluated
Cbaseline (U‡/mL) 0.31 ± 0.20 (n§ = 12) 0.33 ± 0.20 (n = 12) Cmax (U/mL) 0.68 ± 0.08 (n = 12) 0.85 ± 0.12 (n = 13) Tmax (hrs) 3.9 ± 7.3 (n = 12) 2.7 ± 1.9 (n = 13) AUC(0-t) (U hr/mL) 74.5 ± 30.3 (n = 12) 95.9 ± 19.6 (n = 13) CL (mL/min) 0.85 ± 1.07 (n = 7) 1.17 ± 0.78 (n = 9) Half-life (hours) 56 ± 36 (n = 7) 62 ± 38 (n = 9) The maximum plasma concentration (Cmax) and area under the plasma concentration-time curve (AUC) increased from the single to double dose, although the increase was not dose proportional. The mean half-lives of CINRYZE were 56 hours (range 11 to 108 hours) for a single dose and 62 hours (range 16 to 152 hours) for the double dose.
Pharmacokinetics was investigated in children 7 to 11 years old following IV administration of two dose levels of CINRYZE (500 U and 1,000 U) every 3 or 4 days for 12 weeks to prevent angioedema attacks. At one hour post-dose during the steady state (Week 12), mean (SD) C1 inhibitor functional activities were 0.53 (0.13) U/mL, and 0.81 (0.20) U/mL, following IV administration of 500 U and 1,000 U CINRYZE, respectively, increasing from the baseline value of 0.18 (0.10) U/mL.
Population PK modelling was conducted to evaluate the exposure in a pediatric population compared with adults. The PK parameters for functional C1 inhibitor in pediatrics (7 to 11 years) are presented in Table 6. Following dosing every 3 to 4 days with 1,000 U, mean AUC0-4 and Cmax were approximately 30% higher in children 7 to 11 years old than in adults who received 1,000 U.
Table 6 Mean (SD) Steady State PK Parameters of Functional C1 Inhibitor in Pediatrics (7 to 11 years). Parameters 500 U (n=12) 1000 U (n=13) AUC0-4,ss = area under the concentration-time curve from time 0 to 4 h at steady state; AUCtau,ss = area under the concentration-time curve over the dosing interval at steady state; Cmax,ss = maximum concentration at steady state; Cmin,ss = minimum predose concentration at steady state; CL: Clearance. Cmax,ss (U/mL) 0.53 (0.11) 0.89 (0.18) Cmin,ss (U/mL) 0.26 (0.08) 0.34 (0.11) AUCtau,ss (U∙hr/mL) 30.10 (7.57) 45.10 (10.9) AUC0-4,ss (U∙hr/mL) 2.06 (0.42) 3.40 (0.68) CL (mL/min) 0.67 (0.23) 0.67 (0.22) Half-life (hours) 34.60 (6.72) 34.10 (6.67) Studies have not been conducted to evaluate the PK of CINRYZE in special patient populations identified by gender, race, geriatric age, or the presence of renal or hepatic impairment.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No animal studies have been completed to evaluate the effects of CINRYZE on carcinogenesis, mutagenesis, and impairment of fertility.
13.2 Animal Toxicology and/or Pharmacology
Acute toxicity of CINRYZE was studied in a combined acute toxicity and 7-day repeat dose/ dose range finding (DRF) study in Sprague Dawley rats followed by a pivotal 14-day repeat dose study. The acute and 14-day repeat dose toxicity studies were performed with intravenous administration of CINRYZE at dose levels of 1, 7 and 28 times normal dose. No signs of toxicity were observed in the single dose or repeat dose studies. Repeat dosing in the rat resulted in an antibody response between days 1 and 14 that was not characterized for neutralizing activity. However, there was no change in the functional activity of CINRYZE over the dosing period.
In vitro and in vivo animal thrombogenicity studies with CINRYZE showed a potential for clot formation when CINRYZE was administered at doses 14 times the recommended clinical dose (greater than 200U/kg). Animal studies have supported a concern about the risk of thrombosis from intravenous administration of C1 esterase inhibitor products. (see Warnings and Precautions [5.2]).
-
14 CLINICAL STUDIES
The safety and efficacy of CINRYZE prophylaxis therapy to reduce the incidence, severity, and duration of HAE attacks was demonstrated in a single randomized, double blind, placebo controlled multi-center cross-over study of 24 subjects. Subjects were screened to confirm a diagnosis of HAE and a history of at least two HAE attacks per month. 24 subjects (mean age 38.1 years with a range of 9 to 73 years) were randomized to one of two treatment groups: either CINRYZE prophylaxis for 12 weeks followed by 12 weeks of placebo prophylaxis; or randomized to placebo prophylaxis for 12 weeks followed by 12 weeks of CINRYZE prophylaxis. Two subjects dropped out (one in each arm); 22 subjects crossed over into period 2 and were included in the efficacy analysis. Subjects were given blinded injections (CINRYZE or placebo) every 3 to 4 days, approximately 2 times per week. Subjects recorded all angioedema symptoms daily. An attack was defined as the subject-reported indication of swelling at any location following a report of no swelling on the previous day.
The efficacy determination was based on the number of attacks during the 12-week period while receiving CINRYZE as compared to the number of attacks during the placebo treatment period. The effectiveness of C1 esterase inhibitor prophylaxis in reducing the number of HAE attacks was variable among the subjects as shown in Table 7, 8 and 9.
Table 7 Prevention of HAE Attacks by Subject in the Randomized, Placebo-Controlled, Crossover, Routine Prophylaxis Trial. Subject Percent Reduction in Attack Frequency 1 100% 2 100% 3 100% 4 100% 5 90% 6 88% 7 84% 8 83% 9 78% 10 76% 11 60% 12 47% 13 43% 14 43% 15 32% 16 31% 17 25% 18 21% 19 10% 20 1% 21 -8% 22 -85% Table 8 Summary Statistics on Number of HAE Attacks in the Randomized, Placebo-Controlled, Crossover, Routine Prophylaxis Trial. Statistics CINRYZE
N=22Placebo
N=22Mean 6.1 12.7 SD 5.4 4.8 Median 6 13.5 Min 0 6 Max 17 22 Table 9 Summary of Generalized Estimating Equation (GEE) Analysis Results (p-value) in the Randomized, Placebo-Controlled, Crossover, Routine Prophylaxis Trial. Effect Assessed CINRYZE Treatment Effect <0.0001 Sequence Effect 0.3347 Period Effect 0.3494 Subjects treated with CINRYZE had a 66% reduction in days of swelling (p<0.0001), and decreases in the average severity of attacks (p=0.0006) and the average duration of attacks (p=0.0023), as shown in Table 10.
Table 10 Secondary Efficacy Outcomes in the Randomized, Placebo-Controlled, Crossover, Routine Prophylaxis Trial. Parameters CINRYZE
N=22Placebo
N=2295% Confidence Interval for Treatment Effect (Placebo minus Cinryze) - * 1=mild; 2=moderate; and 3=severe
- † p<0.01
Mean Severity of HAE Attacks
(Score from 1 to 3)* (SD)1.3 (0.85) 1.9 (0.36) 0.58† (0.19, 0.97) Mean Duration of HAE Attacks (Days) (SD) 2.1 (1.13) 3.4 (1.4) 1.23† (0.49, 1.96) Days of Swelling (SD) 10.1 (10.73) 29.6 (16.9) 19.5† (11.94, 27.06) The safety and efficacy of CINRYZE (500 U and 1,000 U) for the prevention of HAE attacks and the reduction of the severity and requirement for acute treatment was demonstrated in a randomized, single-blind, multi-center, dose-ranging cross-over study of 12 pediatric subjects aged 7 to 11 years.
During the 12-week study period, a greater reduction in the normalized number of angioedema attacks per month was observed with 1,000 U CINRYZE compared to 500 U CINRYZE (p=0.03). When compared to the baseline observational period, a reduction in the normalized number of angioedema attacks was observed for both CINRYZE 500 U and CINRZYE 1,000 U (mean absolute reduction in number of HAE attacks: 2.6, 3.0 respectively; mean percent reduction in HAE attacks: 71.1% and 84.5%, respectively). In addition, both doses lessened the severity of attacks and reduced the use of acute treatment compared with baseline. Refer to Table 11 and 12 for the additional parameters.
Table 11 Time-Normalized* Number of HAE Attacks per Month during Observation Period and 12 Week Treatment Period with CINRYZE 500 U and 1000 U. Parameters Observation Period
N = 12CINRYZE 500 U
N = 12CINRYZE 1000 U
N = 12- * Scaled normalized score is expressed as the score per month (× 30.4, number of days per month)
Mean (SD) 3.7 (3.15) 1.2 (1.53) 0.7 (1.35) Min, Max 1.0, 11.8 0.0, 5.6 0.0, 4.8 Median 2.2 0.8 0.4 Table 12 Difference in Time-Normalized Number of HAE Attacks per Month for CINRYZE 500 U and 1000 U 12-Week Treatment Periods from Observation Period. Parameters CINRYZE 500 U
N = 12CINRYZE 1000 U
N = 12Mean (SD) -2.6 (2.88) -3.0 (2.87) 90% CI (-4.1, -1.1) (-4.5, -1.5) Mean % reduction 71.1% 84.5% Median % reduction 76.2% 87.4% - 15 REFERENCES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Information for the Patient).
- Inform patients to immediately report the following to their physician:
- Signs of allergic-type hypersensitivity reactions including hives (itchy white elevated patches), tightness of the chest, wheezing, hypotension and anaphylaxis (see Warnings and Precautions [5.1]). Advise patients to discontinue use of CINRYZE and contact their physicians if these symptoms occur.
- Signs of a thromboembolic event including pain and/or swelling of an arm or leg with warmth over the affected area, discoloration of an arm or leg, unexplained shortness of breath, chest pain or discomfort that worsens on deep breathing, unexplained rapid pulse, numbness or weakness on one side of the body.
- Advise patients with known risk factors for thromboembolic events that they may be at increased risk for these events.
- Advise female patients to notify their physician if they become pregnant or intend to become pregnant during their routine prevention with CINRYZE.
- Advise patients to notify their physician if they are breastfeeding or plan to breastfeed.
- Based on their current regimen, advise patients to bring an adequate supply of CINRYZE for routine prevention when traveling.
- Advise patient that, because CINRYZE is made from human blood, it may carry a risk of transmitting infectious agents, e.g. viruses, and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent (see Warnings and Precautions [5.3], see Description [11]). The risk of transmitting disease has been reduced, but not eliminated, by carefully selecting blood donors, testing donors for infections, and inactivating or removing most viruses during the manufacturing process.
- Inform patients of the risks and benefits of CINRYZE before prescribing or administering to the patient.
- Inform patients to immediately report the following to their physician:
-
PATIENT PACKAGE INSERT
FDA-Approved Patient Labeling
Information for the Patient
CINRYZE® (SIN-rise)
(C1 Esterase Inhibitor [Human])
This leaflet summarizes important information about CINRYZE. Please read it carefully before using CINRYZE and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider, and it does not include all of the important information about CINRYZE. If you have any questions after reading this, ask your healthcare provider.
Do not attempt to self-administer unless you have been taught how by your healthcare provider.
What is CINRYZE?
CINRYZE is an injectable medicine that is used to help prevent swelling and/or painful attacks in children (6 years of age and older), teenagers and adults with Hereditary Angioedema (HAE). HAE is caused by the decreased functioning of a protein called C1 esterase inhibitor, that is present in your blood and helps control inflammation (swelling) and parts of the immune system. CINRYZE contains C1 esterase inhibitor. Before you can inject CINRYZE into your vein (intravenous injection), you must dissolve the CINRYZE powder using Sterile Water for Injection, USP. You can get supplies, including Sterile Water for Injection, USP from your pharmacist.
Who should not use CINRYZE?
You should not use CINRYZE if you have had life-threatening immediate hypersensitivity reactions, including anaphylaxis, to the product.
What should I tell my healthcare provider before using CINRYZE?
Tell your healthcare provider about all of your medical conditions, including if you
- have an indwelling catheter/access device in one of your veins.
- have a history of blood clots, heart disease, or stroke.
- are taking birth control pills or androgens.
- are pregnant or planning to become pregnant. It is not known if CINRYZE can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if CINRYZE passes into your milk and if it can harm your baby.
Tell your healthcare provider and pharmacist about all of the medicines you take, including all prescription and non-prescription medicines such as over-the-counter medicines, supplements, or herbal remedies.
What are the possible side effects of CINRYZE?
Allergic reactions may occur with CINRYZE. Call your healthcare provider or get emergency support services right away if you have any of the following symptoms:
- wheezing
- difficulty breathing
- chest tightness
- turning blue (look at lips and gums)
- fast heartbeat
- swelling of the face
- faintness
- rash
- hives
Serious blood clots may occur with CINRYZE. Call your healthcare provider or get emergency support services right away if you have any of the following symptoms:
- pain and/or swelling of an arm or leg with warmth over the affected area
- discoloration of an arm or leg
- unexplained shortness of breath
- chest pain or discomfort that worsens on deep breathing
- unexplained rapid heart rate
- numbness or weakness on one side of the body
The most common side effects seen with CINRYZE were headache, nausea, rash, and vomiting.
These are not all the possible side effects of CINRYZE.
Tell your healthcare provider about any side effect that bothers you or that does not go away. You can also report side effects to Shire Medical Information at 1-800-828-2088 or the FDA at 1-800-FDA-1088.
You can ask your healthcare provider for information that is written for healthcare providers.
How should I store CINRYZE?
Do not freeze CINRYZE.
Store CINRYZE in a refrigerator or at room temperature between 36°F to 77°F (2°C to 25°C).
Keep CINRYZE in the original carton to protect it from light.
Do not use CINRYZE after the expiration date on the vial.
What else should I know about CINRYZE?
Medicines are sometimes prescribed for purposes other than those listed here. Do not use CINRYZE for a condition for which it is not prescribed. Do not share CINRYZE with other people, even if they have the same symptoms that you have.
Because CINRYZE is made from human blood, it may carry a risk of transmitting infectious agents, e.g. viruses, and, theoretically, the Creutzfeldt-Jakob (CJD) agent.
This leaflet summarizes the most important information about CINRYZE. If you would like more information, talk to your healthcare provider. You can ask your healthcare provider or pharmacist for information about CINRYZE that was written for healthcare professionals.
-
Instructions for Use
CINRYZE® (SIN-rise)
(C1 Esterase Inhibitor [Human])
Do not attempt to self-administer unless you have been taught how by your healthcare provider.
See the step-by-step instructions for injecting CINRYZE at the end of this leaflet. You should always follow the specific instructions given by your healthcare provider. The steps listed below are general guidelines for using CINRYZE. If you are unsure of the steps, please call your healthcare provider or pharmacist before using.
Call your healthcare provider right away if swelling is not controlled after using CINRYZE.
Your healthcare provider will prescribe the dose that you should take.
Call your healthcare provider if you take too much CINRYZE.
Call your healthcare provider if you miss a dose of CINRYZE.
Talk to your healthcare provider before traveling. You should plan to bring enough CINRYZE for your treatment during this time.
Preparation of CINRYZE
Always wash your hands before doing the following steps. Try to keep everything clean and germ-free while you are reconstituting CINRYZE. Once you open the vials, you should finish preparing CINRYZE as soon as possible. This will help to keep them germ-free.
CINRYZE is a Freeze-Dried powder that is supplied in a vacuum-sealed vial.
Note: One vial of CINRYZE is required for each 500 U dose. Two vials of CINRYZE are required for each 1,000 U dose. If two vials are required, you should reconstitute both vials according to steps 1 through 6.
Your healthcare provider may decide you require higher doses of CINRYZE. You should always follow the specific instructions given by your healthcare provider.
- 1. Let the vial of CINRYZE and the vial of Sterile Water for Injection, USP (diluent) reach room temperature.
- 2.
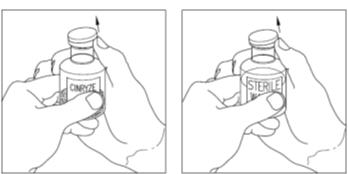
Remove the cap from the vial of CINRYZE and Sterile Water for Injection, USP (diluent) vial to show the center part of the rubber stopper (Figure 6).
Figure 6
- 3.
Wipe the top of each vial with an alcohol wipe or swab, and allow it to dry. Do not blow on the stopper to dry it faster. Place each vial on a flat surface. After cleaning, do not touch the rubber stopper with your hand or allow it to touch any surface (Figure 7).
Figure 7
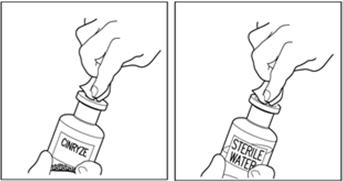
Note: Diluent vial must be penetrated before the CINRYZE vial to prevent loss of vacuum.
- 4.
Remove the protective covering from the top of the Mix2Vial transfer device package (Figure 8). Do not remove the device from the package. Place the Sterile Water for Injection, USP (diluent) vial on a flat surface, and place the blue end of the Mix2Vial transfer device over it, pushing down until the spike penetrates the rubber stopper and the device snaps in place. Mix2Vial transfer device must be positioned completely upright before penetrating the rubber stopper (Figure 9). Remove the plastic package and discard it. Take care not to touch the exposed end of the device (Figure 10).
Figure 8
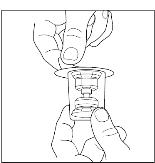
Figure 9
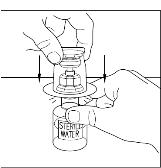
Figure 10
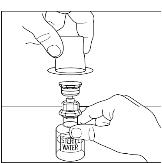
- 5.
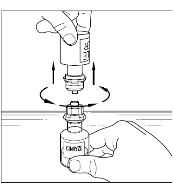
Place the vial of CINRYZE on a flat surface. Turn the diluent vial containing 5 mL of Sterile Water for Injection, USP, upside-down and insert the clear end of the Mix2Vial transfer device into the vial of CINRYZE, pushing down until the spike penetrates the rubber stopper and the device snaps in place (Figure 11). The Mix2Vial transfer device must be positioned completely upright before penetrating the rubber stopper. The Sterile Water for Injection, USP, will automatically flow into the vial of CINRYZE because the vacuum in the vial will draw the Sterile Water for Injection, USP, into the vial of CINRYZE. If this does not happen, do not use the product (Figure 12).
Figure 11
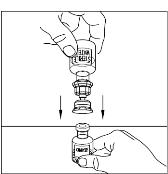
Figure 12
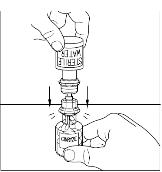
- 6. Once all the Sterile Water for Injection, USP, is in the CINRYZE vial, gently swirl (do not shake) the vial of CINRYZE until all the powder is dissolved (Figure 13). Disconnect the Sterile Water for Injection, USP vial by turning it counterclockwise (Figure 14).
Do not remove the clear end of the Mix2Vial transfer device from the vial of CINRYZE.
Figure 13
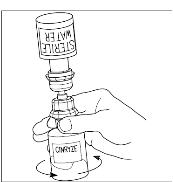
Figure 14
Look at the final solution before using it to make sure that CINRYZE is completely dissolved. The solution should be clear with no evidence of cloudiness. Reconstituted solution should be colorless to slightly blue. Do not use if solution is cloudy or otherwise discolored and call Shire Medical Information at 1-800-828-2088 for further instructions.
One vial of dissolved CINRYZE contains 5 mL of C1 esterase inhibitor at a concentration of 100 U/mL. Prepare one vial of CINRYZE for one 500 U dose. Prepare two vials of CINRYZE for one 1,000 U dose. If preparing two vials, repeat steps 1-6 using a new Mix2Vial transfer device.
Do not reuse the Mix2Vial transfer device. CINRYZE should be administered within 3 hours of reconstitution.
- 7.
Utilizing a sterile, disposable 10mL syringe, draw back the plunger to allow approximately 5mL of air into the syringe. Use of a silicone-free syringe is recommended (Figure 15).
Figure 15
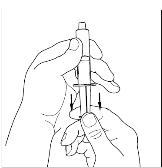
- 8.
Attach the syringe onto the clear end of the Mix2Vial transfer device by turning it clockwise (Figure 16).
Figure 16
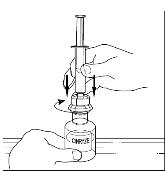
- 9.
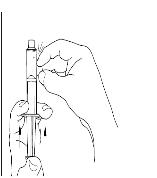
Turn the vial of CINRYZE upside down, inject air into the vial (Figure 17). Slowly pull as much dissolved CINRYZE as possible into the syringe (Figure 18). While holding the vial upside down, detach the syringe from the vial by turning it counterclockwise and releasing it from the Mix2Vial transfer device (Figure 19). Remove any air bubbles by gently tapping the syringe with your finger and slowly pushing the air out of the syringe (Figure 20).
Figure 17
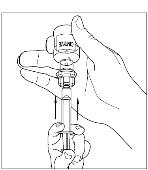
Figure 18
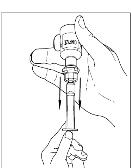
Figure 19
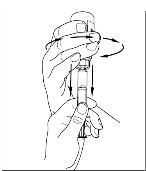
Figure 20
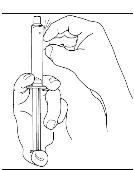
 If preparing a 1,000 U dose (two vials), repeat steps 7 to 9 above with a second vial of CINRYZE to make one complete dose of 10 mL (Figure 21, 22, 23, 24).
If preparing a 1,000 U dose (two vials), repeat steps 7 to 9 above with a second vial of CINRYZE to make one complete dose of 10 mL (Figure 21, 22, 23, 24). - 10. Dispose of the vial(s) with the Mix2Vial transfer device attached to them.
Figure 21
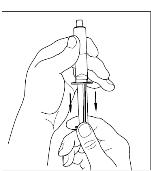
Figure 22
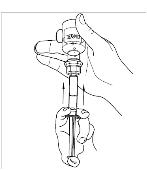
Figure 23
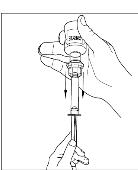
Figure 24
CINRYZE should be administered at room temperature promptly after preparation in the syringe.
SELF ADMINISTRATION (Intravenous Injection)
Your healthcare provider will teach you how to safely administer CINRYZE. It is important that CINRYZE is injected directly into a superficial vein and not injected into surrounding tissues and not injected into an artery. Once you learn how to self-administer, you can follow the instructions in this insert.
- 1. Attach a needle or infusion set with a winged adapter to the syringe containing the dissolved CINRYZE solution. Fill the tubing with dissolved CINRYZE by gently pushing the plunger of the syringe. Be careful not to spill the dissolved CINRYZE. This process replaces the air in the tubing with dissolved CINRYZE.
- 2.
Apply a tourniquet and prepare the injection site by wiping the skin well with an alcohol swab (Figure 25).
Figure 25
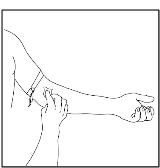
- 3. As instructed by your healthcare provider:
- Insert the butterfly needle of the infusion set tubing into your vein.
- Remove the tourniquet.
- Make sure that the needle is in a vein.
- Inject the dissolved CINRYZE product slowly over five minutes for a 500 U dose, or over ten minutes for a 1,000 U dose (approximately 1mL/min) (Figure 26).
Figure 26
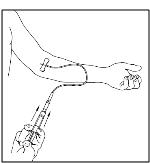
- 4.
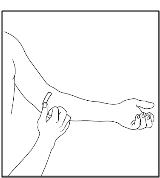
After infusing CINRYZE, remove the infusion set and discard. Cover infusion site with an adhesive bandage (Figure 27). The amount of drug product left in the infusion set will not affect your treatment. Dispose of all unused solution, the empty vial(s), and the used needles and syringe in an appropriate container used for throwing away waste that might hurt others if not handled properly.
Figure 27
It is a good idea to record the lot number from the CINRYZE vial label every time you use CINRYZE.
This Patient Package Insert has been approved by the U.S. Food and Drug Administration.
Manufactured for:
Shire ViroPharma Incorporated
Lexington, MA 02421-2101U.S. License Number 1833
Mix2Vial® is a registered trademark of Medimop Medical Projects, Ltd. in the United States and other jurisdictions.
CINRYZE® is a trademark of Shire ViroPharma Incorporated or its subsidiaries.
©2018 Shire ViroPharma Incorporated. All rights reserved.
-
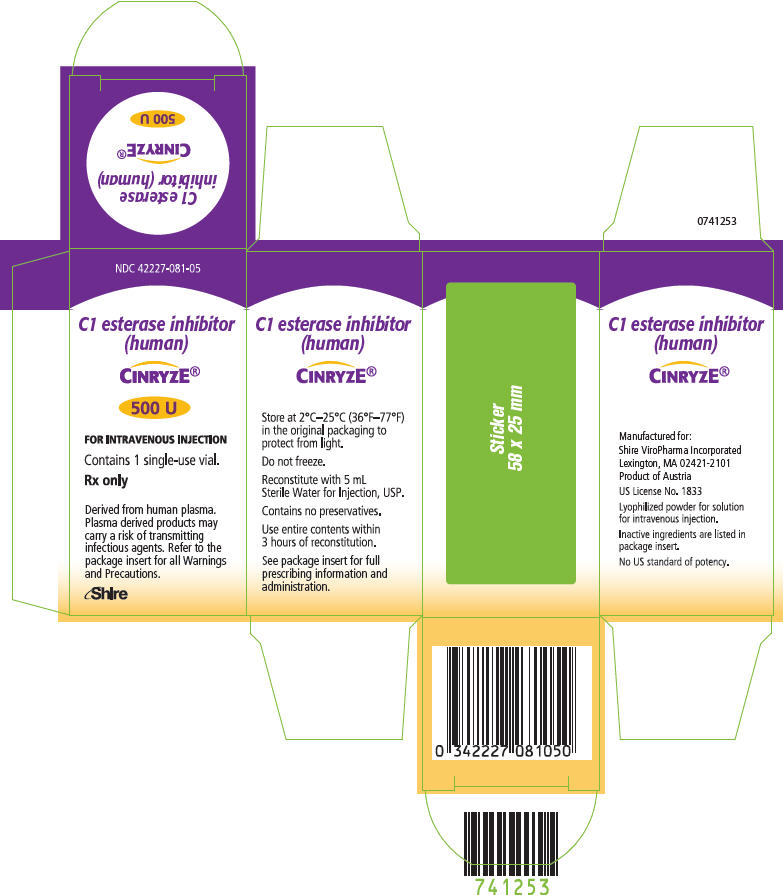
PRINCIPAL DISPLAY PANEL - 500 U Vial Label
FOR INTRAVENOUS INJECTION
C1 esterase inhibitor (human)
CINRYZE®
500 U
NDC: 42227-081-01
Reconstitute with 5 mL Sterile Water for Injection, USP.
Use entire contents within 3 hours of reconstitution.
Single-use vial Rx only US License No. 1833Manufactured for Shire ViroPharma Incorporated, Lexington,
MA 02421-2101.0743288
-
PRINCIPAL DISPLAY PANEL - 500 U Vial Carton
NDC: 42227-081-05
C1 esterase inhibitor
(human)CINRYZE®
500 U
FOR INTRAVENOUS INJECTION
Contains 1 single-use vial.
Rx only
Derived from human plasma.
Plasma derived products may
carry a risk of transmitting
infectious agents. Refer to the
package insert for all Warnings
and Precautions.Shire
-
INGREDIENTS AND APPEARANCE
CINRYZE
human c1-esterase inhibitor injection, powder, lyophilized, for solutionProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 42227-081 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN C1-ESTERASE INHIBITOR (UNII: 6KIC4BB60G) (HUMAN C1-ESTERASE INHIBITOR - UNII:6KIC4BB60G) HUMAN C1-ESTERASE INHIBITOR 500 [iU] in 5 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SUCROSE (UNII: C151H8M554) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) VALINE (UNII: HG18B9YRS7) ALANINE (UNII: OF5P57N2ZX) THREONINE (UNII: 2ZD004190S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42227-081-05 1 in 1 CARTON 1 NDC: 42227-081-01 5 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125267 12/01/2008 Labeler - ViroPharma Biologics, Inc. (832763085) Establishment Name Address ID/FEI Business Operations Sanquin Plasma Products (SPP) 491635727 ANALYSIS Establishment Name Address ID/FEI Business Operations Plasma Industries Belgium SCRL 375250156 ANALYSIS Establishment Name Address ID/FEI Business Operations Baxter Aktiengesellschaft 300434670 LABEL, PACK, MANUFACTURE, ANALYSIS Establishment Name Address ID/FEI Business Operations Baxter Aktiengesellschaft 300466733 ANALYSIS
Trademark Results [Cinryze]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CINRYZE 86743947 not registered Dead/Abandoned |
ViroPharma Biologics, Inc. 2015-09-01 |
 CINRYZE 77644301 3654422 Live/Registered |
VIROPHARMA BIOLOGICS, INC. 2009-01-06 |
 CINRYZE 77212428 3867834 Live/Registered |
VIROPHARMA BIOLOGICS, INC. 2007-06-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.