ANTICOAGULANT CITRATE PHOSPHATE DEXTROSE (CPD) BLOOD-PACK UNITS IN PL 146 PLASTIC (anticoagulant citrate phosphate dextrose- cpd solution solution
Anticoagulant Citrate Phosphate Dextrose (CPD) Blood-Pack Units in PL 146 Plastic by
Drug Labeling and Warnings
Anticoagulant Citrate Phosphate Dextrose (CPD) Blood-Pack Units in PL 146 Plastic by is a Prescription medication manufactured, distributed, or labeled by Fenwal, Inc., Fenwal International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
SPL UNCLASSIFIED SECTION
4R0012MC, 4R0837MC, 4R0112MC
Fenwal Blood-Pack Units
Rx only
With Integral Donor Tube Using ACD or CPD and Fenwal HighFlo Needle
Instructions for Use
Collection Procedure:
Use aseptic technique.
Precaution: Do not use unless solution is clear.
1. Identify Blood-Pack unit using appropriate donor identification system. Confirm that all numbered tubing of each Blood-Pack unit contains its own identical segment numbers.
2. Adjust donor scale to desired collection weight.
3. Position primary container from donor scale as far as possible below donor arm and clamp donor tubing.
4. Following blood center procedures, apply pressure to donor's arm and disinfect site of venipuncture.
5. Remove HighFlo1 needle cover per instructions below:
a) Holding the hub and cover near the tamper-evident seal, twist cover and hub in opposite directions to break seal.
b) Remove needle cover, being careful not to drag the cover across the needle point.
Following blood center procedures, perform venipuncture, appropriately secure donor needle and/or tubing and release clamp.
6. Mix blood and anticoagulant at several intervals during collection and immediately after collection.
7. Collect the appropriate volume based on Blood-Pack unit used.
Note: The volume of anticoagulant is sufficient for the blood collection indicated on Blood-Pack unit ± 10%.
8. Apply clamp to donor tube.
9. As appropriate, release pressure on the donor's arm, collect donor samples following established procedures and withdraw donor needle.
10. Withdrawal of needle into needle guard.
Precaution: The needle guard must be held stationary while the needle is withdrawn into it.
a) Hold sides of needle guard near the front, between the index finger and thumb. Pull the hub back smoothly until the needle is completely enclosed and securely locked into the needle guard.
b) Confirm the needle is completely enclosed and securely locked into the needle guard.
11. Strip blood from donor tubing into container, mix and allow the tubing to refill; repeat once. Seal at X marks on donor tubing to provide numbered aliquots of anticoagulated blood for typing or crossmatching.
12. Discard needle in needle guard into an appropriate biohazardous waste container following established procedures.
13. Store filled unit between 1 and 6° C.
14. Infuse blood within 21 days of collection.
Store at Controlled Room Temperature. Protect from freezing. Avoid excessive heat.
Definition of "Controlled Room Temperature":
"A temperature maintained thermostatically that encompasses the usual and customary working environment of 20° to 25°C (68° to 77°F); that results in a mean kinetic temperature calculated to be not more than 25°C; and that allows for excursions between 15°C and 30°C (59° and 86°F) that are experienced in pharmacies, hospitals, and warehouses. Provided the mean kinetic temperature remains in the allowed range, transient spikes up to 40°C are permitted as long as they do not exceed 24 hours ... The mean kinetic temperature is a calculated value that may be used as an isothermal storage temperature that simulates the non isothermal effects of storage temperature variations."
Reference: United States Pharmacopeia, General Notices. United States Pharmacopeial Convention, Inc.
12601 Twinbrook Parkway, Rockville, MD.
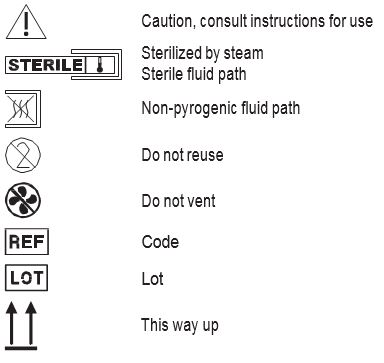
Symbols with Definitions
1 Van der Meer, P.F., & de Korte, D. "Increase of blood donation speed by optimizing the needle-to-tubing connection: an application of donation software." Vox Sanguinis 2009, 97: 21-25

Manufacturer
Fresenius Kabi AG
61346 Bad Homburg / Germany
www.fresenius-kabi.com
1-800-933-6925
© 2019 Fresenius Kabi AG. All rights reserved.
47-23-13-621 REV: A -
PACKAGE/LABEL DISPLAY PANEL
Code 4R0837MC
1 Unit
Fresenius KabiFenwal Blood-Pack Unit
SingleAnticoagulant Citrate Phosphate Dextrose Solution, USP (CPD)
For Collection of 250 mL Blood
Integral Donor Tube, 16 ga. Ultra Thin Wall Fenwal HighFlo NeedleRx only
Each unit consists of a primary container with 35 mL of CPD solution containing 921 mg Sodium Citrate (dihydrate) USP, 893 mg Dextrose (monohydrate) USP, 105 mg Citric Acid (anhydrous) USP, 78 mg Monobasic Sodium Phosphate (monohydrate) USP. pH may have been adjusted with sodium hydroxide.
Sterile, non-pyrogenic fluid path.
See instructions for use.
Single Use OnlyStore at Controlled Room Temperature (refer to direction insert). Protect from freezing. Avoid excessive heat.
- Open pouch by tearing across at notch.
- Direct handling of product surfaces prior to extended storage in the foil pouch, may result in mold growth.
- Unused units in open foil pouch may be kept up to 60 days by folding and securing open end of foil pouch to prevent possible loss of moisture, provided:
- I) Units are not removed from foil pouch, or
- II) Unused units removed from foil pouch are returned to the foil pouch within 12 hours. Units may be removed from the pouch and returned only once.
- Units removed from the foil pouch (that are not returned to the pouch within 12 hours) must be used within 4 days (96 hours). Units out of the foil pouch for longer than 96 hours must be discarded.
Manufacturer
Fresenius Kabi AG
61346 Bad Homburg / Germany
www.fresenius-kabi.com
Made in US
47-28-12-755 REV: A

-
INGREDIENTS AND APPEARANCE
ANTICOAGULANT CITRATE PHOSPHATE DEXTROSE (CPD) BLOOD-PACK UNITS IN PL 146 PLASTIC
anticoagulant citrate phosphate dextrose (cpd) solution solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0942-9201 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) (Anhydrous Citric Acid - UNII:XF417D3PSL) Anhydrous Citric Acid 921 mg in 35 mL DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 893 mg in 35 mL ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) (Anhydrous Citric Acid - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 105 mg in 35 mL SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) (PHOSPHATE ION - UNII:NK08V8K8HR) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE 78 mg in 35 mL Inactive Ingredients Ingredient Name Strength SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0942-9201-01 35 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN170401 03/01/2007 Labeler - Fenwal, Inc. (794519020) Establishment Name Address ID/FEI Business Operations Fenwal International, Inc. 091164590 MANUFACTURE(0942-9201)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.