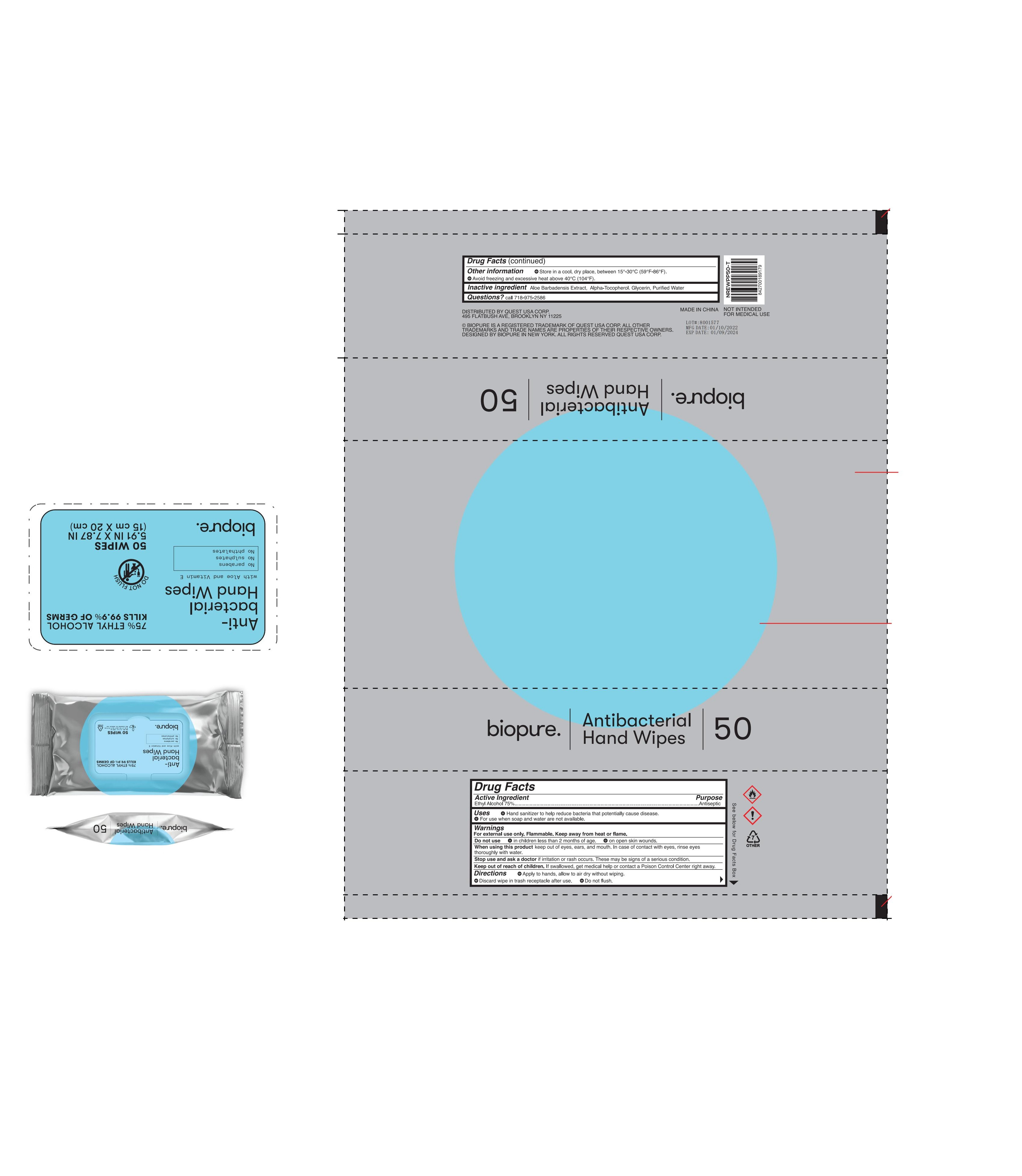
BIOPURE 50CT ANTIBACTERIAL HAND WIPES by Quest USA Corp. / Osike Cosmetics Co., Ltd. 78691-200
BIOPURE 50CT ANTIBACTERIAL HAND WIPES by
Drug Labeling and Warnings
BIOPURE 50CT ANTIBACTERIAL HAND WIPES by is a Otc medication manufactured, distributed, or labeled by Quest USA Corp., Osike Cosmetics Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BIOPURE 50CT ANTIBACTERIAL HAND WIPES- 75% ethyl alcohol wet wipes cloth
Quest USA Corp.
----------
78691-200
Uses
●Hand sanitizer to help reduce bacteria that potentially cause disease.
●For use when soap and water are not available.
Directions
●Apply to hands, allow to air dry without wiping.
●Discard wipe in trash receptacle after use.
● Do not flush.
| BIOPURE 50CT ANTIBACTERIAL HAND WIPES
75% ethyl alcohol wet wipes cloth |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Quest USA Corp. (079869689) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Osike Cosmetics Co., Ltd. | 415803943 | manufacture(78691-200) | |
Revised: 1/2024
Document Id: 0f3069cb-d734-ae08-e063-6294a90a3f0c
Set id: d56f8533-e139-048a-e053-2995a90afa20
Version: 3
Effective Time: 20240117
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.