TROSPIUM CHLORIDE tablet, film coated
Trospium Chloride by
Drug Labeling and Warnings
Trospium Chloride by is a Prescription medication manufactured, distributed, or labeled by Glenmark Pharmaceuticals Inc., USA, Glenmark Pharmaceuticals Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use trospium chloride tablets safely and effectively. See full prescribing information for trospium chloride tablets.
TROSPIUM Chloride Tablets USP, for oral use
Initial U.S. Approval: 2004RECENT MAJOR CHANGES
Warnings and Precautions, Central Nervous System Effects (5.5) 07/2012
INDICATIONS AND USAGE
Trospium chloride tablets USP are a muscarinic antagonist indicated for the treatment of overactive bladder (OAB) with symptoms of urge urinary incontinence, urgency, and urinary frequency. (1)
DOSAGE AND ADMINISTRATION
- The recommended dose of trospium chloride tablets USP is one 20 mg tablet twice daily. Trospium chloride tablets USP should be dosed with water on an empty stomach, at least one hour before a meal. (2)
- For patients with severe renal impairment (creatinine clearance less than 30 mL/min), the recommended dose is 20 mg once daily at bedtime. (2)
- In geriatric patients greater than or equal to 75 years of age, dose may be titrated down to 20 mg once daily based upon tolerability. (2)
DOSAGE FORMS AND STRENGTHS
- 20 mg tablets. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Trospium chloride should be administered with caution to patients with clinically significant bladder outflow obstruction or gastrointestinal obstructive disorders due to risk of urinary or gastric retention. (5.1, 5.3)
- Angioedema of the face, lips, tongue and/or larynx has been reported with trospium chloride. (5.2)
- In patients with controlled narrow angle glaucoma trospium chloride should be used only with careful monitoring. (5.4)
- Central Nervous System Effects: Somnolence has been reported with trospium chloride. Advise patients not to drive or operate heavy machinery until they know how trospium chloride affects them (5.5).
- Trospium is substantially excreted by the kidney. The effects of moderate renal impairment on systemic exposure are not known but systemic exposure is likely increased. Therefore, the risk of anticholinergic adverse reactions is expected to be greater in patients with moderate renal impairment. (5.6)
ADVERSE REACTIONS
The most common adverse reactions (greater than or equal to 1%) with trospium chloride are dry mouth (20.1%), constipation (9.6%), and headache (4.2%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Glenmark Pharmaceuticals Inc., USA at 1(888) 721-7115 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Concomitant use with digoxin did not affect the pharmacokinetics of either drug. (7.1)
- Some drugs which are actively secreted by the kidney may interact with trospium chloride by competing for renal tubular secretion. (7.2)
- Concomitant use with metformin immediate release tablets reduced exposure and peak concentration of trospium. (7.4)
USE IN SPECIFIC POPULATIONS
- The safety and effectiveness of trospium chloride in pediatric patients have not been established. (8.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Urinary Retention
5.2 Angioedema
5.3 Decreased Gastrointestinal Motility
5.4 Controlled Narrow-angle Glaucoma
5.5 Central Nervous System Effects
5.6 Anticholinergic Adverse Reactions in Patients with Moderate Renal Impairment
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-marketing Experience
7 DRUG INTERACTIONS
7.1 Digoxin
7.2 Drugs Eliminated by Active Tubular Secretion
7.3 Antimuscarinic Agents
7.4 Metformin
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 Overdosage
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Angioedema
17.2 When Not to Use
17.3 Administration
17.4 Adverse Reactions
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
The recommended dose is 20 mg twice daily. Trospium chloride tablets USP should be dosed at least one hour before meals or given on an empty stomach.
Dosage modification is recommended in the following patient populations:
- For patients with severe renal impairment (creatinine clearance less than 30 mL/min), the recommended dose is 20 mg once daily at bedtime [see Warnings and Precautions (5.5), Use in Specific Populations (8.6), and Clinical Pharmacology (12.3)].
- In geriatric patients greater than or equal to 75 years of age, dose may be titrated down to 20 mg once daily based upon tolerability [see Use in Specific Populations (8.5)].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Urinary Retention
Trospium chloride should be administered with caution to patients with clinically significant bladder outflow obstruction because of the risk of urinary retention [see Contraindications (4)].
5.2 Angioedema
Angioedema of the face, lips, tongue, and/or larynx has been reported with trospium chloride, the active ingredient in trospium chloride. In one case, angioedema occurred after the first dose of trospium chloride. Angioedema associated with upper airway swelling may be life threatening. If involvement of the tongue, hypopharynx, or larynx occurs, trospium chloride should be promptly discontinued and appropriate therapy and/or measures necessary to ensure a patent airway should be promptly provided.
5.3 Decreased Gastrointestinal Motility
Trospium chloride should be administered with caution to patients with gastrointestinal obstructive disorders because of the risk of gastric retention [see Contraindications (4)]. Trospium chloride, like other antimuscarinic agents, may decrease gastrointestinal motility and should be used with caution in patients with conditions such as ulcerative colitis, intestinal atony and myasthenia gravis.
5.4 Controlled Narrow-angle Glaucoma
In patients being treated for narrow-angle glaucoma, trospium chlorideshould only be used if the potential benefits outweigh the risks and in that circumstance only with careful monitoring [see Contraindications (4)].
5.5 Central Nervous System Effects
Trospium chloride is associated with anticholinergic central nervous system (CNS) effects [see Adverse Reactions (6.2)]. A variety of CNS anticholinergic effects have been reported, including dizziness, confusion, hallucinations and somnolence. Patients should be monitored for signs of anticholinergic CNS effects, particularly after beginning treatment or increasing the dose. Advise patients not to drive or operate heavy machinery until they know how trospium chloride affects them. If a patient experiences anticholinergic CNS effects, dose reduction or drug discontinuation should be considered.
5.6 Anticholinergic Adverse Reactions in Patients with Moderate Renal Impairment
Trospium is substantially excreted by the kidney. The effects of moderate renal impairment on systemic exposure are not known but systemic exposure is likely increased. Therefore, anticholinergic adverse reactions (including dry mouth, constipation, dyspepsia, urinary tract infection, and urinary retention) are expected to be greater in patients with moderate renal impairment [see Dosage and Administration (2), and Use in Specific Populations (8.6)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of trospium chloride was evaluated in controlled clinical trials in a total of 2975 patients, who were treated with trospium chloride (N=1673), placebo (N=1056) or active control medications (N=246). Of this total, 1181 patients participated in two, 12-week, U.S., efficacy and safety studies and a 9-month open-label extension. Of this total, 591 patients received trospium chloride tablets 20 mg twice daily. In all controlled trials combined, 232 and 208 patients received treatment with trospium chloride for at least 24 and 52 weeks, respectively.
In all placebo-controlled trials combined, the incidence of serious adverse events was 2.9% among patients receiving trospium chloride tablets 20 mg twice daily and 1.5% among patients receiving placebo. Table 1 lists adverse reactions from the combined 12-week U.S. safety and efficacy trials were reported by at least 1% of patients, and were reported more frequently in the trospium chloride group than in the placebo group.
The two most common adverse reactions reported by patients receiving trospium chloride tablets 20 mg twice daily were dry mouth and constipation. The single most frequently reported adverse reaction for trospium chloride, dry mouth, occurred in 20.1% of trospium chloride treated patients and 5.8% of patients receiving placebo. In the two U.S. studies, dry mouth led to discontinuation in 1.9% of patients treated with trospium chloride tablets 20 mg twice daily. For the patients who reported dry mouth, most had their first occurrence of the event within the first month of treatment.
Table 1. Incidence (%) of adverse reactions with trospium chloride, reported in greater than or equal to 1% of all patients treated with trospium chloride and more frequent with trospium chloride tablets (20 mg twice daily) than placebo in Studies 1 and 2 combined
Adverse Reaction
Placebo
(N=590)
Trospium Chloride Tablets20 mg
twice daily
(N=591)
Gastrointestinal Disorders
Dry mouth
34 ( 5.8)
119 (20.1)
Constipation
27 (4.6)
57 (9.6)
Abdominal pain upper
7 (1.2)
9 (1.5)
Constipation aggravated
5 (0.8)
8 (1.4)
Dyspepsia
2 (0.3)
7 (1.2)
Flatulence
5 (0.8)
7 (1.2)
Nervous System Disorders
Headache
12 (2.0)
25 (4.2)
General Disorders
Fatigue
8 (1.4)
11 (1.9)
Renal and Urinary Disorders
Urinary retention
2 (0.3)
7 (1.2)
Eye Disorders
Dry eyes
2 (0.3)
7 (1.2)
Other adverse reactions from the U.S., placebo-controlled trials, occurring in greater than or equal to 0.5% and less than 1.0% of trospium chloride treated patients, and more common with trospium chloride than placebo are: tachycardia, vision blurred, abdominal distension, vomiting, dysgeusia, dry throat, and dry skin.
During controlled clinical studies, one adverse reaction of angioneurotic edema was reported.
6.2 Post-marketing Experience
The following adverse reactions have been identified during post-approval use of trospium chloride. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal – gastritis; Cardiovascular – palpitations, supraventricular tachycardia, chest pain, syncope, “hypertensive crisis”; Immunological – Stevens-Johnson syndrome, anaphylactic reaction, angioedema; Nervous System – dizziness, confusion, vision abnormal, hallucinations, somnolence and delirium; Musculoskeletal – rhabdomyolysis; General – rash.
-
7 DRUG INTERACTIONS
7.1 Digoxin
Concomitant use of trospium chloride and digoxin did not affect the pharmacokinetics of either drug [see Clinical Pharmacology (12.3)].
7.2 Drugs Eliminated by Active Tubular Secretion
Although demonstrated in a drug-drug interaction study not to affect the pharmacokinetics of digoxin, trospium chloride has the potential for pharmacokinetic interactions with other drugs that are eliminated by active tubular secretion (e.g., procainamide, pancuronium, morphine, vancomycin, and tenofovir). Coadministration of trospium chloride with these drugs may increase the serum concentration of trospium chloride and/or the coadministered drug due to competition for this elimination pathway. Careful patient monitoring is recommended in patients receiving such drugs [see Clinical Pharmacology (12.3)].
7.3 Antimuscarinic Agents
The concomitant use of trospium chloride with other antimuscarinic agents that produce dry mouth, constipation, and other anticholinergic pharmacological effects may increase the frequency and/or severity of such effects. Trospium chloride may potentially alter the absorption of some concomitantly administered drugs due to anticholinergic effects on gastrointestinal motility.
7.4 Metformin
Co-administration of 500 mg metformin immediate release tablets twice daily with trospium chloride 60 mg extended release reduced the steady-state systemic exposure of trospium by approximately 29% for mean AUC0-24 and by 34% for mean Cmax [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects
Pregnancy Category C: There are no adequate and well-controlled studies of trospium chloride in pregnant women. Trospium chlorideshould be used during pregnancy only if the potential benefit to the patient outweighs the risk to the patient and fetus. Women who become pregnant during trospium chloride treatment are encouraged to contact their physician.
Risk Summary
Based on animal data, trospium chloride is predicted to have a low probability of increased risk of adverse developmental outcomes, above background risk. Adverse developmental findings were not observed to correlate with dose in rats or in rabbits. No increased risk above background was observed in rats and rabbits treated at an exposure approximately equivalent to the maximal recommended human dose (MRHD) of 40 mg.
Animal Data
In a rat embryo/fetal development study, pregnant rats received doses of trospium chloride up to 200 mg/kg/day, from implantation to closure of the fetal hard palate, with maternal systemic exposures corresponding to approximately nine times the exposure of women treated at the MRHD of 40 mg, based on AUC. No malformations or fetal toxicity were observed.
The offspring of female rats exposed orally, pre-and post-natally, to trospium chloride up to 200 mg/kg/day showed no increased developmental toxicity over background in surviving pups. However, maternal toxicity (death, irregular breathing, increased excitability) was observed at 200 mg/kg/day. A no-effect level for maternal and pup toxicity (survival to Day 4) was 20 mg/kg/day, an exposure approximately equivalent to the maximal recommended human dose (MRHD) of 40 mg.
In a rabbit embryo/fetal development study, pregnant rabbits received doses of trospium chloride up to 200 mg/kg/day, from implantation to closure of the fetal hard palate. At 200 mg/kg/day, maternal systemic exposures corresponded to approximately 16 times the exposure of women treated at the MRHD of 40 mg, based on AUC. However, one fetus in each of the three treated dose groups (0.3 to 16 times exposures at the MRHD) demonstrated multiple malformations, including umbilical hernia and skeletal malformations. A maternal no-effect level was set at 20 mg/kg/day, at an exposure approximately equivalent to the maximal recommended human dose (MRHD) of 40 mg, due to clinical signs (reduced feces, hunched posture, diarrhea) observed in a pharmacokinetic study at 200 mg/kg/day.
8.3 Nursing Mothers
Trospium chloride (2 mg/kg orally and 50 mcg/kg intravenously) was excreted, to a limited extent (less than 1%), into the milk of lactating rats (primarily as parent compound). It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, trospium chloride should be used during lactation only if the potential benefit justifies the potential risk to the newborn.
8.4 Pediatric Use
The safety and effectiveness of trospium chloride in pediatric patients have not been established.
8.5 Geriatric Use
Of the 591 patients with overactive bladder who received treatment with trospium chloride in the two U.S., placebo-controlled, efficacy and safety studies, 249 patients (42%) were 65 years of age and older. Eighty-eight trospium chloride treated patients (15%) were greater than or equal to 75 years of age.
In these 2 studies, the incidence of commonly reported anticholinergic adverse reactions in patients treated with trospium chloride (including dry mouth, constipation, dyspepsia, urinary tract infection, and urinary retention) was higher in patients 75 years of age and older as compared to younger patients. This effect may be related to an enhanced sensitivity to anticholinergic agents in this patient population [see Clinical Pharmacology (12.3)]. Therefore, based upon tolerability, the dose frequency of trospium chloride may be reduced to 20 mg once daily in patients 75 years of age and older.
8.6 Renal Impairment
Severe renal impairment (creatinine clearance less than 30 mL/minute) significantly altered the disposition of trospium chloride. A 4.2-fold and 1.8-fold increase in mean AUC(0-∞) and Cmax, respectively, and the appearance of an additional elimination phase with a long half-life (~33 hr) were detected in patients with severe renal impairment compared with nearly age-matched subjects with creatinine clearance equal to or higher than 80 mL/min. The different pharmacokinetic behavior of trospium chloride in patients with severe renal impairment necessitates adjustment of dosage frequency [see Dosage and Administration (2)]. The pharmacokinetics of trospium have not been studied in patients with creatinine clearance ranging from 30-80 mL/min.
Trospium is known to be substantially excreted by the kidney, and the risk of adverse reactions may be greater in patients with impaired renal function.
8.7 Hepatic Impairment
There is no information regarding the effect of severe hepatic impairment on exposure to trospium chloride. In a study of patients with mild and with moderate hepatic impairment, given 40 mg of immediate-release trospium chloride, mean Cmax increased 12% and 63%, respectively, and mean AUC(0-∞) decreased 5% and 15%, respectively, compared to healthy subjects. The clinical significance of these findings is unknown. Caution should be used when administering trospium chloride to patients with moderate and severe hepatic impairment.
-
10 Overdosage
Overdosage with antimuscarinic agents, including trospium chloride, can result in severe antimuscarinic effects. Supportive treatment should be provided according to symptoms. In the event of overdosage, electrocardiographic monitoring is recommended.
A 7-month-old baby experienced tachycardia and mydriasis after administration of a single dose of trospium 10 mg given by a sibling. The baby’s weight was reported as 5 kg. Following admission into the hospital and about 1 hour after ingestion of the trospium, medicinal charcoal was administered for detoxification. While hospitalized, the baby experienced mydriasis and tachycardia up to 230 beats per minute. Therapeutic intervention was not deemed necessary. The baby was discharged as completely recovered the following day.
-
11 DESCRIPTION
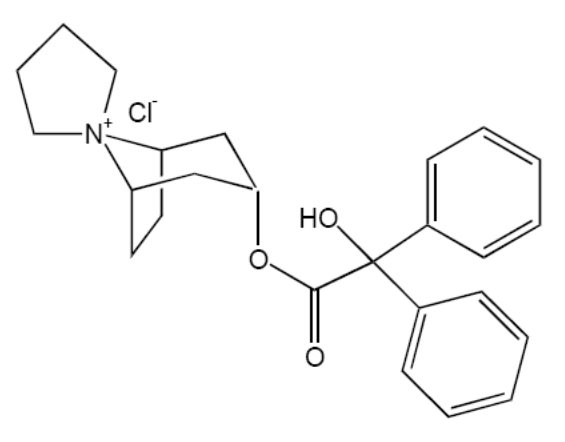
Trospium chloride USP is a quaternary ammonium compound with the chemical name of Spiro[8-azoniabicyclo[3.2.1]octane-8,1'-pyrrolidinium], 3-[(hydroxydiphenylacetyl)oxy]-, chloride, (1α, 3β, 5α). The empirical formula of trospium chloride USP is C25H30ClNO3 and its molecular weight is 427.97. The structural formula of trospium chloride USP is represented below:
Trospium chloride USP is a white or almost white, crystalline powder. It is soluble in water, freely soluble in methanol, practically insoluble in methylene chloride.
Each trospium chloridetablet USP contains 20 mg of trospium chloride USP, a muscarinic antagonist, for oral administration. Each tablet also contains the following inactive ingredients: microcrystalline cellulose, lactose monohydrate, corn starch, povidone, croscarmellose sodium, colloidal silicon dioxide, magnesium stearate, sucrose, copovidone, titanium dioxide, polyethylene glycol 6000, talc, hypromellose, macrogol, yellow iron oxide, red iron oxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Trospium chloride is a muscarinic antagonist.
Trospium chloride antagonizes the effect of acetylcholine on muscarinic receptors in cholinergically innervated organs including the bladder. Its parasympatholytic action reduces the tonus of smooth muscle in the bladder.
Receptor assays showed that trospium chloride has negligible affinity for nicotinic receptors as compared to muscarinic receptors at concentrations obtained from therapeutic doses.
12.2 Pharmacodynamics
Placebo-controlled studies employing urodynamic variables were conducted in patients with conditions characterized by involuntary detrusor contractions. The results demonstrate that trospium chlorideincreases maximum cystometric bladder capacity and volume at first detrusor contraction.
Electrophysiology
The effect of 20 mg twice daily and up to 100 mg twice daily trospium chloride on QT interval was evaluated in a single-blind, randomized, placebo and active (moxifloxacin 400 mg once daily) controlled 5 day parallel trial in 170 male and female healthy volunteer subjects aged 18 to 45 years. The QT interval was measured over a 24-hour period at steady state. The 100 mg twice daily dose of trospium chloride was chosen because this achieves the Cmax expected in severe renal impairment. Trospium chloride was not associated with an increase in individual corrected (QTcI) or Fridericia corrected (QTcF) QT interval at any time during steady state measurement, while moxifloxacin was associated with a 6.4 msec increase in QTcF.
In this study, asymptomatic, non-specific T wave inversions were observed more often in subjects receiving trospium chloride than in subjects receiving moxifloxacin or placebo following five days of treatment. This finding was not observed during routine safety monitoring in 2 other placebo-controlled clinical trials in 591 trospium chloride treated overactive bladder patients [see Clinical Studies (14)]. The clinical significance of T wave inversion in this study is unknown. Trospium chloride is associated with an increase in heart rate that correlates with increasing plasma concentrations. In the study described above, trospium chloride demonstrated a mean increase in heart rate compared to placebo of 9.1 bpm for the 20 mg dose and of 18 bpm for the 100 mg dose. In the two U.S. placebo-controlled trials in patients with overactive bladder, the mean increase in heart rate compared to placebo in Study 1 was observed to be 3 bpm and in Study 2 was 4 bpm.
12.3 Pharmacokinetics
Absorption:
After oral administration, less than 10% of the dose is absorbed. Mean absolute bioavailability of a 20 mg dose is 9.6% (range: 4-16.1%). Peak plasma concentrations (Cmax) occur between 5 to 6 hours post-dose. Mean Cmax increases greater than dose-proportionally; a 3-fold and 4-fold increase in Cmax was observed for dose increases from 20 mg to 40 mg and from 20 mg to 60 mg, respectively. AUC exhibits dose linearity for single doses up to 60 mg. Trospium chloride exhibits diurnal variability in exposure with a decrease in Cmax and AUC of up to 59% and 33%, respectively, for evening relative to morning doses.
Effect of Food:
Administration with a high (50%) fat-content meal resulted in reduced absorption, with AUC and Cmax values 70-80% lower than those obtained when trospium chloride was administered while fasting. Therefore, it is recommended that trospium chlorideshould be taken at least one hour prior to meals or on an empty stomach [see Dosage and Administration (2)].
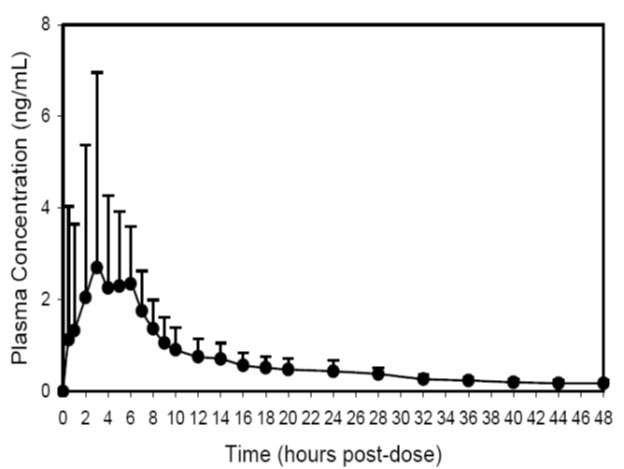
A summary of mean (± standard deviation) pharmacokinetic parameters for a single 20 mg dose of trospium chloride tablets is provided in Table 2.
Table 2. Mean (± SD) Pharmacokinetic Parameter Estimates for a Single 20 mg Trospium Chloride Tablet Dose in Healthy Volunteers
Cmax
AUC0-∞
Tmax
t½
(ng/mL)
(ng/mLhr)
(hr)
(hr)
3.5 ± 4.0
36.4 ± 21.8
5.3 ± 1.2
18.3 ± 3.2
The mean plasma concentration-time (+ SD) profile for trospium chloride is shown in Figure 1.
Figure 1 -Mean (+ SD) Concentration-Time Profile for a Single 20 mg Oral Dose of Trospium Chloride Tablets in Healthy Volunteers
Distribution:
Protein binding ranged from 50 to 85% when concentration levels of trospium chloride (0.5-50 ng/mL) were incubated with human serum in vitro.
The 3H-trospium chloride ratio of plasma to whole blood was 1.6:1. This ratio indicates that the majority of 3H-trospium chloride is distributed in plasma.
The apparent volume of distribution for a 20 mg oral dose is 395 (± 140) liters.
Metabolism:
The metabolic pathway of trospium in humans has not been fully defined. Of the 10% of the dose absorbed, metabolites account for approximately 40% of the excreted dose following oral administration. The major metabolic pathway is hypothesized as ester hydrolysis with subsequent conjugation of benzylic acid to form azoniaspironortropanol with glucuronic acid. Cytochrome P450 (CYP) is not expected to contribute significantly to the elimination of trospium. Data taken from in vitro human liver microsomes investigating the inhibitory effect of trospium on seven CYP isoenzyme substrates (CYP1A2, 2A6, 2C9, 2C19, 2D6, 2E1, and 3A4) suggest a lack of inhibition at clinically relevant concentrations.
Excretion:
The plasma half-life for trospium chloride following oral administration is approximately 20 hours. After oral administration of an immediate-release formulation of 14C-trospium chloride, the majority of the dose (85.2%) was recovered in feces and a smaller amount (5.8% of the dose) was recovered in urine; 60% of the radioactivity excreted in urine was unchanged trospium.
The mean renal clearance for trospium (29.07 L/hour) is 4-fold higher than average glomerular filtration rate, indicating that active tubular secretion is a major route of elimination for trospium. There may be competition for elimination with other compounds that are also renally eliminated [see Drug Interactions (7.2)].
Drug Interactions
Digoxin: Concomitant use of 20 mg trospium chloride immediate release twice daily at steady state and a single dose of 0.5 mg digoxin in a crossover study with 40 male and female subjects did not affect the pharmacokinetics of either drug.
Metformin: A drug interaction study was conducted in which trospium chloride extended release 60 mg once daily was coadministered with Glucophage® (metformin hydrochloride) 500 mg twice daily under steady-state conditions in 44 healthy subjects. Co-administration of 500 mg metformin immediate release tablets twice daily reduced the steady-state systemic exposure of trospium by approximately 29% for mean AUC0-24 and by 34% for mean Cmax. The effect of decrease in trospium exposure on the efficacy of trospium chloride extended release is unknown. The steady-state pharmacokinetics of metformin were comparable when administered with or without 60 mg trospium chloride extended release once daily under fasted condition. The effect of metformin at higher doses on trospium PK is unknown.
Specific Populations
Age: Age did not appear to significantly affect the pharmacokinetics of trospium chloride, however, increased anticholinergic side effects unrelated to drug exposure were observed in patients greater than or equal to 75 years of age [see Use in Specific Populations (8.5)].
Pediatric: The pharmacokinetics of trospium chloride were not evaluated in pediatric patients.
Race: Pharmacokinetic differences due to race have not been studied.
Gender: Studies comparing the pharmacokinetics in different genders had conflicting results. When a single 40 mg trospium chloride tablets dose was administered to 16 elderly subjects, exposure was 45% lower in elderly females compared to elderly males. When 20 mg trospium chloride tablets was dosed twice daily for 4 days to 6 elderly males and 6 elderly females (60 to 75 years), AUC and Cmax were 26% and 68% higher, respectively, in females without hormone replacement therapy than in males.
Renal Impairment: In a clinical pharmacokinetic study where a single dose of 40 mg immediate release trospium chloride was administered to 12 healthy males and 12 males with severe renal impairment, severe renal impairment (creatinine clearance less than 30 mL/minute) significantly altered the disposition of trospium chloride. A 4.2-fold and 1.8-fold increase in mean AUC(0-∞) and Cmax, respectively, and the appearance of an additional elimination phase with a long half-life (~33 hours vs. 18 hours) were detected in patients with severe renal impairment compared with nearly age-matched subjects with creatinine clearance equal to or higher than 80 mL/min. The different pharmacokinetic behavior of trospium chloridein patients with severe renal impairment necessitates adjustment of dosage frequency [see Dosage and Administration (2)]. The pharmacokinetics of trospium have not been studied in patients with creatinine clearance ranging from 30-80 mL/min.
Hepatic Impairment: In a clinical pharmacokinetic study in patients with mild (Child-Pugh score 5-6) and with moderate (Child-Pugh score 7-8) hepatic impairment, given a single dose of 40 mg immediate-release trospium chloride, mean Cmax increased 12% and 63%, respectively, and mean AUC(0-∞) decreased 5% and 15%, respectively, compared to healthy subjects. There is no information regarding the effect of severe hepatic impairment on exposure to trospium chloride.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: Carcinogenicity studies with trospium chloride were conducted in mice and rats for 78 weeks and 104 weeks, respectively, at maximally tolerated doses. No evidence of a carcinogenic effect was found in either mice or rats administered up to 200 mg/kg/day, approximately 9 times the expected clinical exposure levels at the maximum recommended human dose (MRHD) of 40 mg.
Mutagenesis: Trospium chloride was not mutagenic nor genotoxic in tests in vitro in bacteria (Ames test) and mammalian cells (L5178Y mouse lymphoma and CHO cells) or in vivo in the rat micronucleus test.
Impairment of Fertility: No evidence of impaired fertility was observed in rats administered doses up to 200 mg/kg/day (about 16 times the expected clinical exposure at the MRHD, based on AUC).
-
14 CLINICAL STUDIES
Trospium chloride was evaluated for the treatment of patients with overactive bladder who had symptoms of urinary frequency, urgency, and urge incontinence in two U.S. 12-week, placebo-controlled studies and one 9-month open label extension.
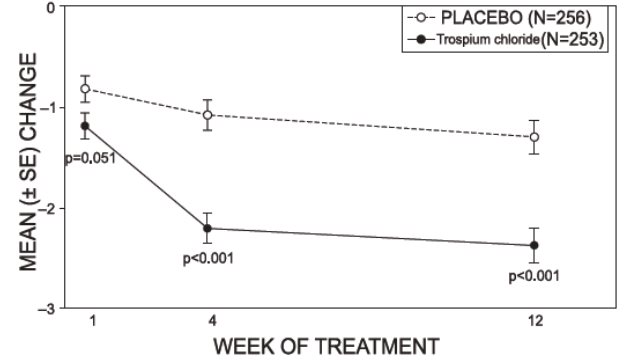
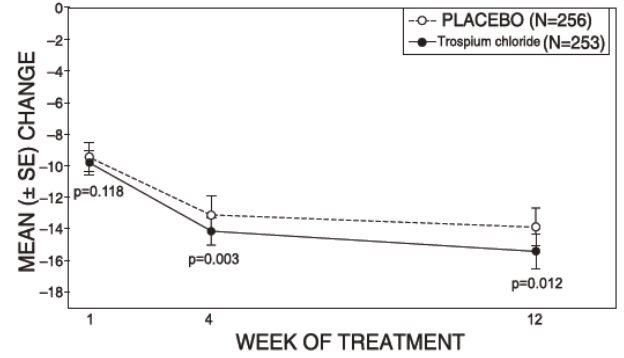
Study 1 was a randomized, double-blind, placebo-controlled, parallel-group study in 523 patients. A total of 262 patients received trospium chloride tablets 20 mg twice daily and 261 patients received placebo. The majority of patients were Caucasian (85%) and female (74%) with a mean age of 61 years (range: 21 to 90 years). Entry criteria required that patients have urge or mixed incontinence (with a predominance of urge), urge incontinence episodes of at least 7 per week, and greater than 70 micturitions per week. The patient’s medical history and urinary diary during the treatment-free baseline confirmed the diagnosis. Reductions in urinary frequency, urge incontinence episodes and urinary void volume for placebo and trospium chloride treatment groups are summarized in Table 3 and Figures 2 and 3.
Table 3. Mean (SE) change from baseline to end of treatment (Week 12 or last observation carried forward) for urinary frequency, urge incontinence episodes, and void volume in Study 1
Efficacy endpoint
Placebo
N=256
Trospium Chloride
N=253
P-value
Urinary frequency/24 hours a,*
Mean baseline
12.9
12.7
Mean change from baseline
-1.3 (0.2)
-2.4 (0.2)
<0.001
Urge incontinence episodes/week b,*
Mean baseline
30.1
27.3
Mean change from baseline
-13.9 (1.2)
-15.4 (1.1)
0.012
Urinary void volume/toilet void (mL)a,c
Mean baseline
156.6
155.1
Mean change from baseline
7.7 (3.1)
32.1 (3.1)
<0.001
a Treatment differences assessed by analysis of variance for ITT:LOCF data set.
b Treatment differences assessed by ranked analysis of variance for ITT:LOCF data set.
c Placebo N=253, trospium chloride N=248.
* Denotes co-primary endpoint
ITT=intent-to-treat, LOCF=last observation carried forward.
Figure 2 – Mean Change from Baseline in Urinary Frequency/24 Hours, by Visit: Study 1
Figure 3 – Mean Change from Baseline in Urge Incontinence/Week, by Visit: Study 1
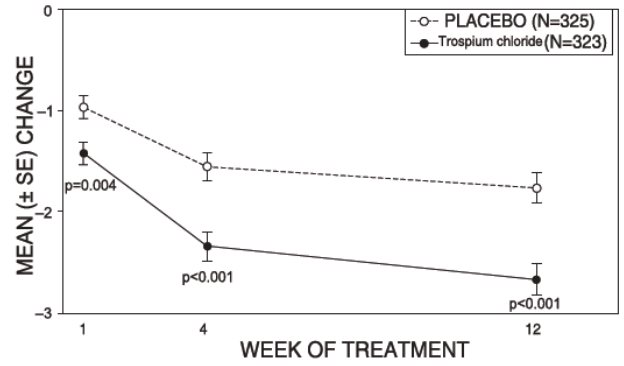
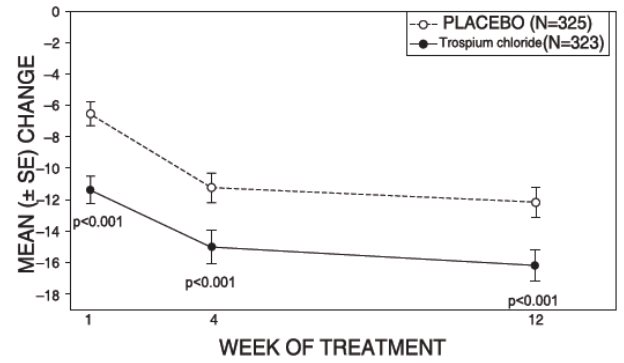
Study 2 was nearly identical in design to Study 1. A total of 329 patients received trospium chloride tablets 20 mg twice daily and 329 patients received placebo. The majority of patients were Caucasian (88%) and female (82%) with a mean age of 61 years (range: 19 to 94 years). Entry criteria were identical to Study 1. Reductions in urinary frequency, urge incontinence episodes, and urinary void volume for placebo and trospium chloride treatment groups are summarized in Table 4 and Figures 4 and 5.
Table 4. Mean (SE) change from baseline to end of treatment (Week 12 or last observation carried forward) for urinary frequency, urge incontinence episodes, and void volume in Study 2
Efficacy endpoint
Placebo
N=325
Trospium Chloride
N=323
P-value
Urinary frequency/24 hours a,*
Mean baseline
13.2
12.9
Mean change from baseline
-1.8 (0.2)
-2.7 (0.2)
<0.001
Urge incontinence episodes/week b
Mean baseline
27.3
26.9
Mean change from baseline
-12.1 (1.0)
-16.1 (1.0)
<0.001
Urinary void volume/toilet void (mL)a,c
Mean baseline
154.6
154.8
Mean change from baseline
9.4 (2.8)
35.6 (2.8)
<0.001
a Treatment differences assessed by analysis of variance for ITT:LOCF data set.
b Treatment differences assessed by ranked analysis of variance for ITT:LOCF data set.
c Placebo N=320, trospium chloride N=319.
* Denotes primary endpoint
ITT=intent-to-treat, LOCF=last observation carried forward.
Figure 4 – Mean Change from Baseline in Urinary Frequency/24 Hours, by Visit: Study 2
Figure 5 – Mean Change from Baseline in Urge Incontinence/Week, by Visit: Study 2
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Trospium chloride tablets USP 20 mg (brownish yellow, round, biconvex film-coated tablets, debossed with ‘L’ on one side and ‘1’ on other side) are supplied as follows:
Bottles of 30 NDC: 68462-461-30
Bottles of 60 NDC: 68462-461-60
Bottles of 500 NDC: 68462-461-05
Bottles of 1000 NDC: 68462-461-10
-
17 PATIENT COUNSELING INFORMATION
“See FDA-approved Patient Labeling (Patient Information)”
17.1 Angioedema
Patients should be informed that trospium chloride, the active ingredient in trospium chloride tablets, may produce angioedema which could result in life-threatening airway obstruction. Patients should be advised to promptly discontinue trospium chloride tablets and seek immediate medical attention if they experience edema of the tongue, edema of the laryngopharynx, or difficulty breathing.
17.2 When Not to Use
Prior to treatment, patients should fully understand the risks and benefits of trospium chloride. In particular, patients should be informed not to take trospium chloride tablets if they:
- have urinary retention;
- gastric retention;
- uncontrolled narrow-angle glaucoma;
- are allergic to any component of trospium chloride tablets.
17.3 Administration
Patients should be instructed regarding the recommended dosing and administration of trospium chloride tablets:
- Take one trospium chloride tablet twice daily with water.
- Take trospium chloride tablets on an empty stomach or at least 1 hour before a meal.
17.4 Adverse Reactions
Patients should be informed that the most common side effects with trospium chloride are dry mouth and constipation and that other less common side effects include trouble emptying the bladder, blurred vision, and heat prostration. Because anticholinergics, such as trospium chloride, may produce dizziness or blurred vision, patients should be advised to exercise caution in decisions to engage in potentially dangerous activities until the drug’s effects have been determined. Patients should be informed that alcohol may enhance the drowsiness caused by anticholinergic agents.
-
PATIENT INFORMATION
Trospium Chloride Tablets USP
Read the Patient Information that comes with trospium chloride tablets before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your medical condition or your treatment.
What are trospium chloride tablets?
Trospium chloride tablets are a prescription medicine used to treat adults with overactive bladder who have the following symptoms:
- a strong need to urinate right away;
- leaking or wetting accidents due to a strong need to urinate right away;
- a need to urinate often.
Who should not take trospium chloride tablets?
Do not take trospium chloride tablets if you:
- have trouble emptying your bladder;
- have delayed or slow emptying of your stomach;
- have an eye problem called “uncontrolled narrow-angle glaucoma”;
- are allergic to trospium chloride tablets or any of its ingredients. See the end of this leaflet for a complete list of ingredients.
Trospium chloride tablets have not been studied in children under the age of 18 years.
What should I tell my doctor before starting trospium chloride tablets?
Tell your doctor about all of your medical conditions including if you:
- have any stomach or intestinal problems or problems with constipation;
- have trouble emptying your bladder or have a weak urine stream;
- have an eye problem called narrow-angle glaucoma;
- have kidney problems;
- have liver problems;
- are pregnant or planning to become pregnant. It is not known if trospium chloride tablets can harm your unborn baby.
- are breastfeeding. It is not known if trospium chloride passes into breast milk and if it can harm your baby. You should talk to your doctor about the best way to feed your baby if you are taking trospium chloride tablets.
Tell your doctor about all the medicines you take including prescription and nonprescription medicines, vitamins and herbal supplements. Trospium chloride tablets and certain other medicines can interact and make some side effects worse. Trospium chloride tablets can affect how other medicines are handled by the body.
Know all the medicines you take. Keep a list of them with you to show your doctor and pharmacist each time you get a new medicine.
How should I take trospium chloride tablets?
Take trospium chloride tablets exactly as prescribed.
- Take one trospium chloride tablet twice daily with water.
- Take trospium chloride tablets on an empty stomach or at least 1 hour before a meal.
- If you take too much trospium chloride call your local Poison Control Center or go to an emergency room right away.
What are the possible side effects of trospium chloride tablets?
Trospium chloride tablets may cause allergic reactions that may be serious. Symptoms of a serious allergic reaction may include swelling of the face, lips, throat or tongue. If you experience these symptoms, you should stop taking trospium chloride tablets and get emergency medical help right away.
The most common side effects with trospium chloride tablets are:
- dry mouth;
- constipation;
- headache.
Trospium chloride tablets may cause other less common side effects, including:
- trouble emptying the bladder;
- blurred vision; and drowsiness. Do not drive or operate heavy machinery until you know how trospium chloride tablets affect you. Alcohol can worsen the drowsiness caused by drugs such as trospium chloride tablets.
- heat prostration. Due to decreased sweating, heat prostration can occur when drugs such as trospium chloride tablets are used in a hot environment.
Tell your doctor if you have any side effects that bother you or that do not go away.
These are not all possible side effects of trospium chloride tablets. For more information, ask your doctor, healthcare professional or pharmacist.
How should I store trospium chloride tablets?
- Keep trospium chloride tablets and all other medicines out of the reach of children.
- Store trospium chloride tablets at room temperature, 68° to 77°F (20° to 25°C). Protect from light.
- Safely dispose of trospium chloride tablets that are out of date or that you no longer need.
General information about trospium chloride tablets
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use trospium chloride tabletsfor a condition for which they were not prescribed. Do not give trospium chloride tablets to other people, even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about trospium chloride tablets. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about trospium chloride tablets that is written for health professionals. You can also call Glenmark Pharmaceuticals Inc., USA at 1 (888)721-7115.
What are the ingredients in trospium chloride tablets?
Active Ingredient: trospium chloride USP.
Inactive Ingredients: microcrystalline cellulose, lactose monohydrate, corn starch, povidone, croscarmellose sodium, colloidal silicon dioxide, magnesium stearate, sucrose, copovidone, titanium dioxide, polyethylene glycol 6000, talc, hypromellose, macrogol, yellow iron oxide, red iron oxide.
Rx only
Manufactured by:
Glenmark Pharmaceuticals Ltd.
IndiaGlenmark Pharmaceuticals Inc., USA
Mahwah, NJ 07430Questions? 1 (888)721-7115
www.glenmarkpharma.com/usaDecember 2014
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC: 68462-461-60
TROSPIUM CHLORIDE TABLETS USP
20 mg
Pharmacist: Dispense the patient information sheet provided separately to each patient. -
INGREDIENTS AND APPEARANCE
TROSPIUM CHLORIDE
trospium chloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68462-461 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TROSPIUM CHLORIDE (UNII: 1E6682427E) (TROSPIUM - UNII:T4Y8ORK057) TROSPIUM CHLORIDE 20 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) SUCROSE (UNII: C151H8M554) COPOVIDONE K25-31 (UNII: D9C330MD8B) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 6000 (UNII: 30IQX730WE) TALC (UNII: 7SEV7J4R1U) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color YELLOW (Brownish yellow) Score no score Shape ROUND (Biconvex) Size 6mm Flavor Imprint Code L;1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68462-461-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 08/13/2010 2 NDC: 68462-461-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 08/13/2010 3 NDC: 68462-461-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 08/13/2010 4 NDC: 68462-461-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 08/13/2010 03/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091575 08/13/2010 Labeler - Glenmark Pharmaceuticals Inc., USA (130597813) Establishment Name Address ID/FEI Business Operations Glenmark Pharmaceuticals Limited 677318665 ANALYSIS(68462-461) , MANUFACTURE(68462-461) Establishment Name Address ID/FEI Business Operations Glenmark Pharmaceuticals Limited 862603186 ANALYSIS(68462-461) , MANUFACTURE(68462-461) Establishment Name Address ID/FEI Business Operations Glenmark Life Sciences Limited 650283737 API MANUFACTURE(68462-461)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.