GENICIN VITA-D- folic acid, cholecalciferol, folate tablet
Genicin Vita-D by
Drug Labeling and Warnings
Genicin Vita-D by is a Other medication manufactured, distributed, or labeled by 7T Pharma LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DOSAGE & ADMINISTRATION
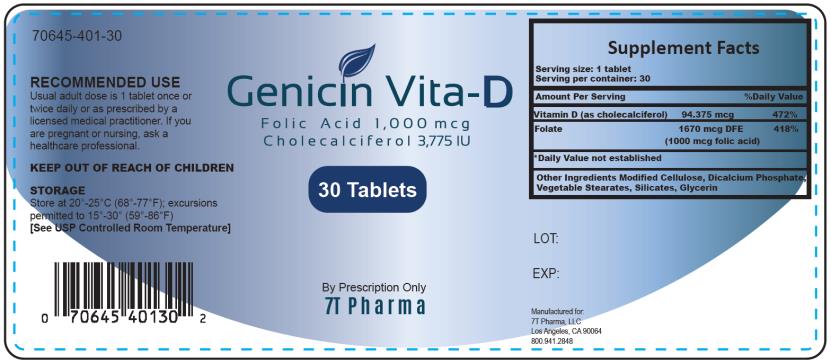
RECOMMENDED DOSAGE:
Usual adult dose is 1 tablet once or twice daily or as prescribed by a licensed medical practitioner. If you are pregnant or nursing, ask a healthcare professional.Genicin Vita-D contains the following inactive ingredients: Modified Cellulose, Dicalcium Phosphate, Vegetable Stearates, Silicates, and Glycerin.
- Genicin Vita-D
-
WARNINGS:
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.
Genicin Vita-D tablets should only be used under the direction and supervision of a licensed medical practitioner. Use with caution in patients that may have a medical condition, are pregnant, lactating, trying to conceive, under the age of 18, or taking medications.
-
PRECAUTIONS:
KEEP OUT OF REACH OF CHILDREN.
Tamper Evident: Do not use if seal is broken or missing.
Tell your doctor if you have: kidney problems, thyroid disease. This medication should be used as directed during pregnancy or while breast-feeding. Consult your doctor about the risks and benefits.
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid supplementation may obscure pernicious anemia, in that hematologic remission can occur while neurological manifestations progress.Genicin Vita-D is supplied in:
30ct bottles (70645-401-30*)
Dispensed by Prescription†
-
Dietary Supplement
Store at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F) [See USP Controlled Room Temperature.]
Protect from heat, light and moisture.* 7T Pharma does not represent these product codes to be National Drug Codes (NDC). Product codes are formatted according to standard industry practice, to meet the formatting requirement by pedigree reporting and supply-chain control including pharmacies.
† This product is a prescription-folate with or without other dietary ingredients that – due to increased folate levels increased risk associated with masking of B12 deficiency (pernicious anemia) requires administration under the care of a licensed medical practitioner (61 FR 8760).1-3 The most appropriate way to ensure pedigree reporting consistent with these regulatory guidelines and safety monitoring is to dispense this product only by prescription (Rx). This is not an Orange Book product. This product may be administered only under a physician’s supervision and all prescriptions using this product shall be pursuant to state statutes as applicable. The ingredients, indication or claims of this product are not to be construed to be drug claims.
- Federal Register Notice of August 2, 1973 (38 FR 20750)
- Federal Register Notice of October 17, 1980 (45 FR 69043, 69044)
- Federal Register Notice of March 5, 1996 (61 FR 8760)
Genicin Vita-D Tablets
Manufactured for:
7T Pharma, LLC
Los Angeles, CA 90064
800.941.2848 - Federal Register Notice of August 2, 1973 (38 FR 20750)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GENICIN VITA-D
folic acid, cholecalciferol, folate tabletProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:70645-401 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1000 ug CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 94.375 ug Inactive Ingredients Ingredient Name Strength HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) HYDRATED SILICA (UNII: Y6O7T4G8P9) GELATIN (UNII: 2G86QN327L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:70645-401-30 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 10/01/2018 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color scoring 1 shape size (solid drugs) 5 mm Labeler - 7T Pharma LLC (080220022)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.