EPOPROSTENOL - epoprostenol injection, powder, lyophilized, for solution
Sun Pharmaceutical Industries, Inc.
----------
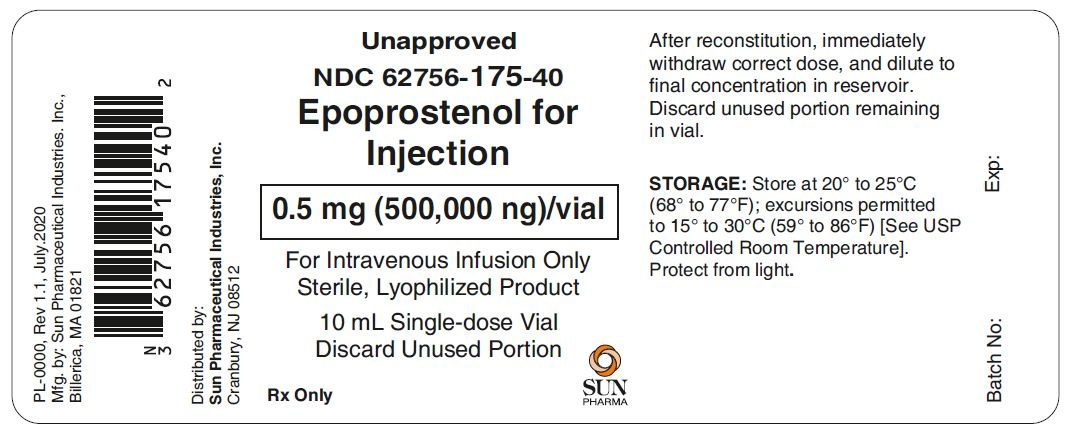
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 0.5 mg-label
Unapproved
NDC: 62756-175-40
Epoprostenol for Injection
0.5 mg (500,000 ng)/vial
For Intravenous Infusion Only
Sterile, Lyophilized Product
10 mL Single-dose Vial
Discard Unused Portion
Rx only
Sun Pharma
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 0.5 ng Carton
Unapproved
NDC: 62756-175-40
Epoprostenol for Injection
0.5 mg (500,000 ng)/vial
For Intravenous Infusion Only
Sterile, Lyophilized Product
1 x 10 mL Single-dose Vial
Discard Unused Portion
Rx only
Sun Pharma

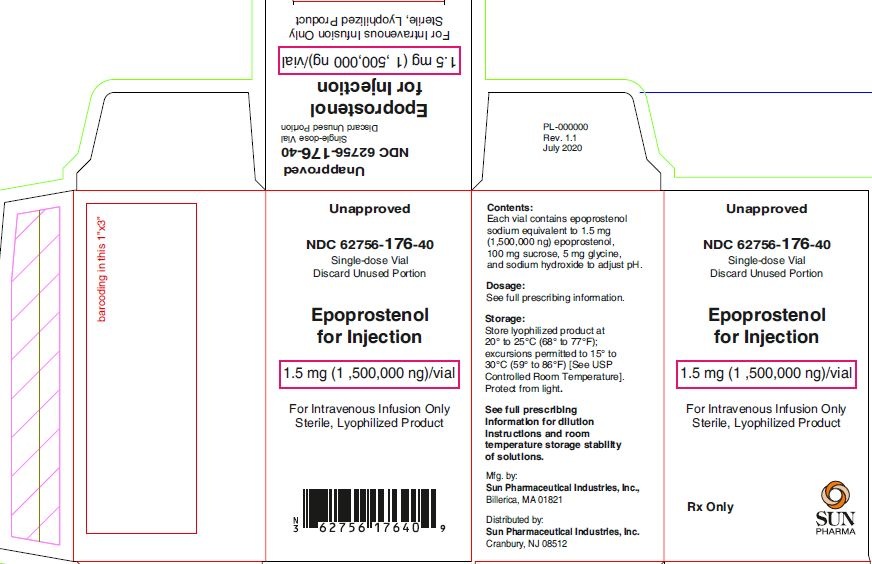
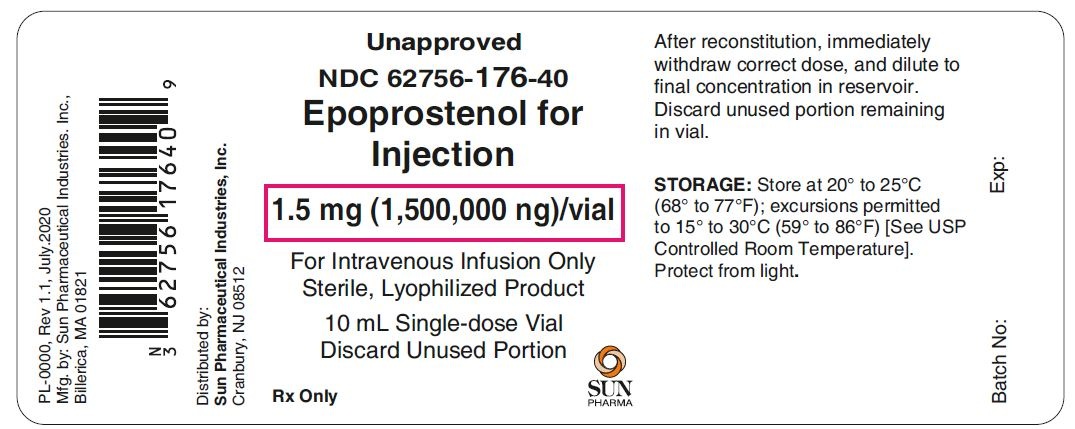
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 1.5 ng Label
Unapproved
NDC: 62756-176-40
Epoprostenol for Injection
1.5 mg (1,500,000 ng)/vial
For Intravenous Infusion Only
Sterile, Lyophilized Product
10 mL Single-dose Vial
Discard Unused Portion
Rx only
Sun Pharma
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 1.5 ng Carton
Unapproved
NDC: 62756-176-40
Epoprostenol for Injection
1.5 mg (1,500,000 ng)/vial
For Intravenous Infusion Only
Sterile, Lyophilized Product
1 x 10 mL Single-dose Vial
Discard Unused Portion
Rx only
Sun Pharma