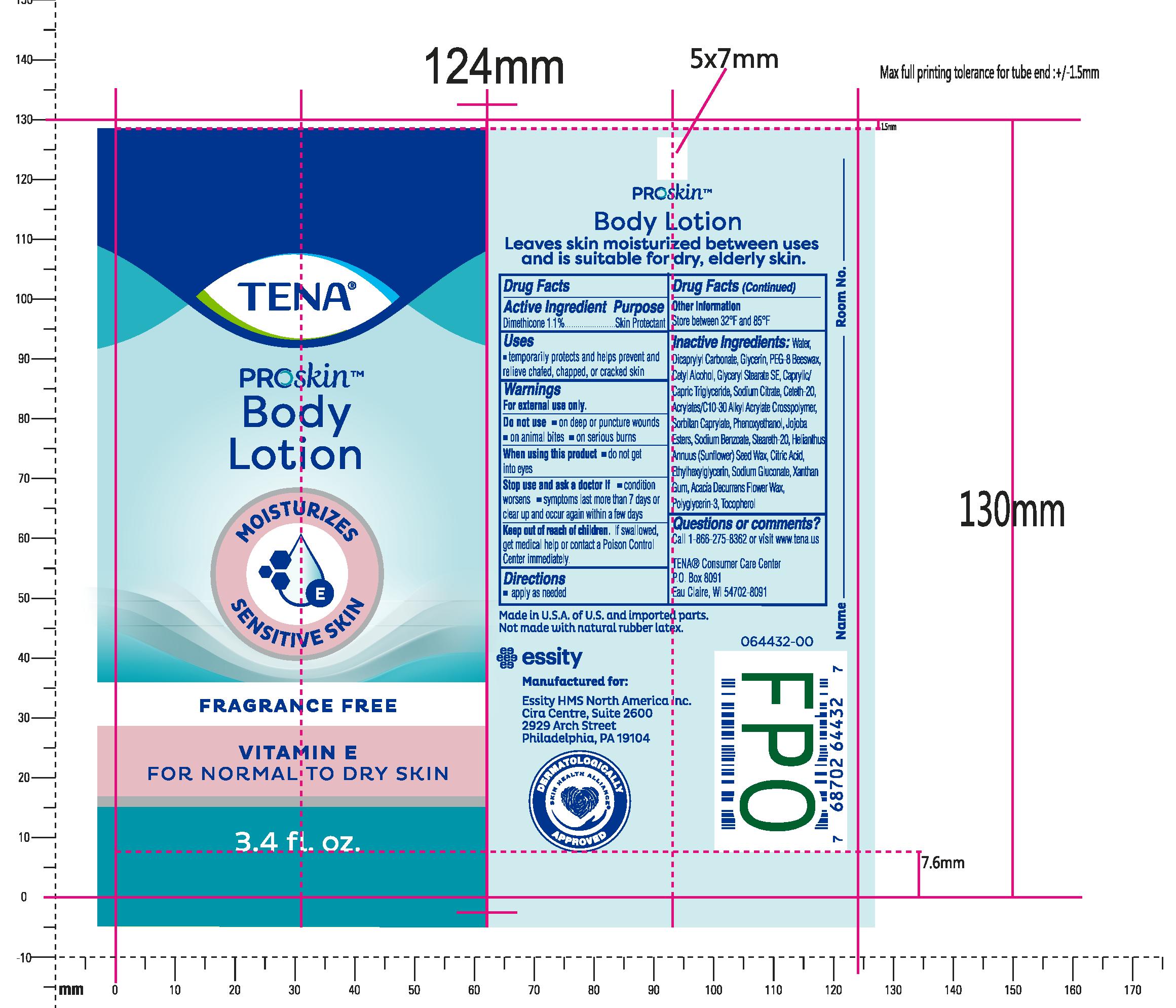
Essity (as PLD) - TENA PROSKIN BODY LOTION (59608-009)
TENA PROSKIN FRAGRANCE-FREE BODY by
Drug Labeling and Warnings
TENA PROSKIN FRAGRANCE-FREE BODY by is a Otc medication manufactured, distributed, or labeled by ESSITY HMS NORTH AMERICA INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
TENA PROSKIN FRAGRANCE-FREE BODY- dimethicone lotion
ESSITY HMS NORTH AMERICA INC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Essity (as PLD) - TENA PROSKIN BODY LOTION (59608-009)
WARNINGS
FOR EXTERNAL USE ONLY.
DO NOT USE
- ON DEEP OR PUNCTURE WOUNDS
- ON ANIMAL BITES
- ON SERIOUS BURNS
WHEN USING THIS PRODUCT
- DO NOT GET INTO EYES.
STOP USE AND ASK A DOCTOR IF
- CONDITION WORSENS
- SYMPTOMS LAST MORE THAN 7 DAYS OR CLEAR UP AND OCCUR AGAINWITHIN A FEW DAYS.
KEEP OUT OF REACH OF CHILDREN. IF SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.
INACTIVE INGREDIENTS
WATER, DICAPRYLYL CARBONATE, PEG-8 BEESWAX, CETYL ALCOHOL, GLYCERYL STEARATE, CAPRYLIC/CAPRIC TRIGLYCERIDE, SODIUM CITRATE, CETETH-20, ACRYLATES C10-30 ALKYL ACRYLATE CROSSPOLYMER, SODIUM CAPRYLATE, PHENOXYETHANOL, JOJOBA ESTERS, SODIUM BENZOATE, STEARETH-20, HELIANTHUS ANNUUS (SUNFLOWER) SEED WAX, CITRIC ACID, ETHYLHEXYLGLYCERIN, SODIUM GLUCONATE, XANTHAN GUM, ACACIA DECURRENS FLOWER WAX, POLYGLYCERIN-3, TOCOPHEROL
| TENA PROSKIN FRAGRANCE-FREE BODY
dimethicone lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - ESSITY HMS NORTH AMERICA INC (787850148) |