COMPLERA- emtricitabine, rilpivirine hydrochloride, and tenofovir disoproxil fumarate tablet, film coated
COMPLERA by
Drug Labeling and Warnings
COMPLERA by is a Prescription medication manufactured, distributed, or labeled by Gilead Sciences, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use COMPLERA safely and effectively. See full prescribing information for COMPLERA.
COMPLERA® (emtricitabine, rilpivirine, tenofovir disoproxil fumarate) tablets, for oral use
Initial U.S. Approval: 2011WARNING: POSTTREATMENT ACUTE EXACERBATION OF HEPATITIS B
See full prescribing information for complete boxed warning.
Severe acute exacerbations of hepatitis B virus (HBV) have been reported in patients coinfected with HIV-1 and HBV who have discontinued products containing emtricitabine (FTC) and/or tenofovir disoproxil fumarate (TDF), two of the components of COMPLERA. Closely monitor hepatic function with both clinical and laboratory follow-up for at least several months in patients who are coinfected with HIV-1 and HBV and discontinue COMPLERA. If appropriate, initiation of anti-hepatitis B therapy may be warranted. (5.1)
RECENT MAJOR CHANGES
Warnings and Precautions Immune Reconstitution Syndrome (5.9) 11/2019 INDICATIONS AND USAGE
COMPLERA, a combination of two nucleoside analog HIV-1 reverse transcriptase inhibitors (emtricitabine and tenofovir disoproxil fumarate) and one non-nucleoside reverse transcriptase inhibitor (rilpivirine), is indicated for use as a complete regimen for the treatment of HIV-1 infection in patients weighing at least 35 kg (1) as initial therapy in those with no antiretroviral treatment history and with HIV-1 RNA less than or equal to 100,000 copies/mL at the start of therapy, or (2) or to replace a stable antiretroviral regiment in those who are virologically suppressed (HIV-1 RNA <50 copies/mL) on a stable antiretroviral regimen for at least 6 months with no treatment failure and no known substitutions associated with resistance to the individual components of COMPLERA. (1, 14)
Limitations of Use:
DOSAGE AND ADMINISTRATION
- Testing: Prior to or when initiating COMPLERA, test for hepatitis B virus infection. Prior to initiation and during treatment with COMPLERA, on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose, and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorus. (2.1)
- Recommended dosage in adults and pediatric patients weighing at least 35 kg: One tablet taken orally once daily with food. (2.2)
- For pregnant patients who are already on COMPLERA prior to pregnancy and who are virologically suppressed (HIV-1 RNA less than 50 copies per mL), one tablet taken once daily may be continued. Lower exposures of rilpivirine were observed during pregnancy; therefore, viral load should be monitored closely. (2.3)
- Renal impairment: Not recommended in patients with estimated creatinine clearance below 50 mL per minute. (2.4)
- Recommended dosage with rifabutin coadministration: an additional 25 mg tablet of rilpivirine (Edurant) once per day taken concomitantly with COMPLERA and with a meal for the duration of the rifabutin coadministration. (2.5, 7.6, 12.3)
DOSAGE FORMS AND STRENGTHS
Tablets: 200 mg of emtricitabine, 25 mg of rilpivirine, and 300 mg of tenofovir disoproxil fumarate. (3)
CONTRAINDICATIONS
COMPLERA is contraindicated when coadministered with drugs which may result in loss of virologic response and possible resistance to COMPLERA. (4)
WARNINGS AND PRECAUTIONS
- Skin and Hypersensitivity Reactions: Severe skin and hypersensitivity reactions have been reported during postmarketing experience, including cases of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS). Immediately discontinue treatment if hypersensitivity or rash with systemic symptoms or elevations in hepatic serum biochemistries develops and closely monitor clinical status, including hepatic serum biochemistries. (5.2)
- Hepatotoxicity: Hepatic adverse events have been reported in patients receiving a rilpivirine-containing regimen. Monitor liver-associated tests before and during treatment with COMPLERA in patients with underlying hepatic disease or marked elevations in liver-associated tests. Also consider monitoring liver-associated tests in patients without risk factors. (5.3)
- Depressive disorders: Severe depressive disorders have been reported. Immediate medical evaluation is recommended for severe depressive disorders. (5.4)
- New onset or worsening renal impairment: Can include acute renal failure and Fanconi syndrome. Avoid administering COMPLERA with concurrent or recent use of nephrotoxic drugs. (5.5)
- Decreases in bone mineral density (BMD): Consider monitoring BMD in patients with a history of pathologic fracture or other risk factors of osteoporosis or bone loss. (5.6)
- Concomitant use of COMPLERA with drugs with a known risk to prolong the QTc interval of the electrocardiogram may increase the risk of Torsade de Pointes. (5.7)
- Lactic acidosis/severe hepatomegaly with steatosis: Discontinue treatment in patients who develop symptoms or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity. (5.8)
- Immune reconstitution syndrome: May necessitate further evaluation and treatment. (5.9)
ADVERSE REACTIONS
- Most common adverse reactions to rilpivirine (incidence greater than or equal to 2%, Grades 2–4) are depressive disorders, insomnia, and headache. (6.1)
- Most common adverse reactions to emtricitabine and tenofovir disoproxil fumarate (incidence greater than or equal to 10%) are diarrhea, nausea, fatigue, headache, dizziness, depression, insomnia, abnormal dreams, and rash. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Gilead Sciences, Inc. at 1-800-GILEAD-5 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
- COMPLERA is a complete regimen for the treatment of HIV-1 infection; therefore, coadministration with other antiretroviral medications for treatment of HIV-1 infection is not recommended. (7.1)
- Consult the Full Prescribing Information prior to and during treatment for important drug interactions. (4, 5.7, 7)
USE IN SPECIFIC POPULATIONS
- Pregnancy: Monitor viral load closely during pregnancy as rilpivirine exposures were generally lower during pregnancy. (2.3, 8.1, 12.3)
- Lactation: Breastfeeding not recommended due to the potential for HIV-1 transmission. (8.2)
- Pediatrics: Not recommended for patients weighing less than 35 kg. (8.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: POSTTREATMENT ACUTE EXACERBATION OF HEPATITIS B
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Testing Prior to Initiation and During Treatment with COMPLERA
2.2 Recommended Dosage
2.3 Recommended Dosage During Pregnancy
2.4 Not Recommended in Patients with Moderate or Severe Renal Impairment
2.5 Recommended Dosage with Rifabutin Coadministration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Severe Acute Exacerbation of Hepatitis B in Patients Coinfected with HIV-1 and HBV
5.2 Skin and Hypersensitivity Reactions
5.3 Hepatotoxicity
5.4 Depressive Disorders
5.5 New Onset or Worsening Renal Impairment
5.6 Bone Loss and Mineralization Defects
5.7 Risk of Adverse Reactions or Loss of Virologic Response Due to Drug Interactions
5.8 Lactic Acidosis/Severe Hepatomegaly with Steatosis
5.9 Immune Reconstitution Syndrome
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Not Recommended with Other Antiretroviral Medications
7.2 Drugs Inducing or Inhibiting CYP3A Enzymes
7.3 Drugs Increasing Gastric pH
7.4 Drugs Affecting Renal Function
7.5 QT Prolonging Drugs
7.6 Significant Drug Interactions
7.7 Drugs with No Observed Interactions with COMPLERA
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Adult Subjects
14.2 Pediatric Subjects
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: POSTTREATMENT ACUTE EXACERBATION OF HEPATITIS B
Severe acute exacerbations of hepatitis B virus (HBV) have been reported in patients who are coinfected with HBV and HIV-1 and have discontinued products containing emtricitabine (FTC) and/or tenofovir disoproxil fumarate (TDF), two of the components of COMPLERA.
Closely monitor hepatic function with both clinical and laboratory follow-up for at least several months in patients who are coinfected with HIV-1 and HBV and discontinue COMPLERA. If appropriate, initiation of anti-hepatitis B therapy may be warranted [see Warnings and Precautions (5.1)].
-
1 INDICATIONS AND USAGE
COMPLERA® is indicated as a complete regimen for the treatment of HIV-1 infection in adults and pediatric patients weighing at least 35 kg:
- as initial therapy in those with no antiretroviral treatment history with HIV-1 RNA less than or equal to 100,000 copies/mL at the start of therapy or
- to replace a stable antiretroviral regimen in those who are virologically suppressed (HIV-1 RNA less than 50 copies/mL) on a stable antiretroviral regimen for at least 6 months with no treatment failure and no known substitutions associated with resistance to the individual components of COMPLERA [see Microbiology (12.4) and Clinical Studies (14)].
Limitations of Use:
- More rilpivirine-treated subjects with HIV-1 RNA greater than 100,000 copies/mL at the start of therapy experienced virologic failure (HIV-1 RNA ≥50 copies/mL) compared to rilpivirine-treated subjects with HIV-1 RNA less than or equal to 100,000 copies/mL [see Clinical Studies (14)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Testing Prior to Initiation and During Treatment with COMPLERA
Prior to or when initiating COMPLERA, test patients for hepatitis B virus infection [see Warnings and Precautions (5.1)].
Prior to initiation of COMPLERA, and during treatment with COMPLERA, on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorus [see Warnings and Precautions (5.5)].
2.2 Recommended Dosage
COMPLERA is a three-drug fixed dose combination product containing 200 mg of emtricitabine (FTC), 25 mg of rilpivirine (RPV), and 300 mg of tenofovir disoproxil fumarate (TDF). The recommended dosage of COMPLERA in adult and pediatric patients weighing at least 35 kg is one tablet taken orally once daily with food [see Use in Specific Populations (8.4) and Clinical Pharmacology (12.3)].
2.3 Recommended Dosage During Pregnancy
For pregnant patients who are already on COMPLERA prior to pregnancy and are virologically suppressed (HIV-1 RNA less than 50 copies per mL), one tablet of COMPLERA taken once daily may be continued. Lower exposures of rilpivirine, a component of COMPLERA, were observed during pregnancy, therefore viral load should be monitored closely [see Use in Specific Populations (8.1) and Clinical Pharmacology (12.3)].
2.4 Not Recommended in Patients with Moderate or Severe Renal Impairment
COMPLERA is not recommended in patients with moderate or severe renal impairment (estimated creatinine clearance below 50 mL per minute) [see Warnings and Precautions (5.5) and Use in Specific Populations (8.6)].
2.5 Recommended Dosage with Rifabutin Coadministration
If COMPLERA is coadministered with rifabutin, take an additional 25 mg tablet of rilpivirine (Edurant®) with COMPLERA once daily with a meal for the duration of the rifabutin coadministration [see Drug Interactions (7.6) and Clinical Pharmacology (12.3)].
-
3 DOSAGE FORMS AND STRENGTHS
Each COMPLERA tablet contains 200 mg of emtricitabine (FTC), 27.5 mg of rilpivirine hydrochloride (equivalent to 25 mg of rilpivirine [RPV]), and 300 mg of tenofovir disoproxil fumarate (TDF, equivalent to 245 mg of tenofovir disoproxil).
The tablets are purplish pink, capsule shaped, film coated, debossed with "GSI" on one side, and plain faced on the other side.
-
4 CONTRAINDICATIONS
COMPLERA is contraindicated when coadministered with the following drugs; coadministration may result in loss of virologic response and possible resistance to COMPLERA or to the class of NNRTIs [see Warnings and Precautions (5.7), Drug Interactions (7), and Clinical Pharmacology (12.3)]:
- Anticonvulsants: carbamazepine, oxcarbazepine, phenobarbital, phenytoin
- Antimycobacterials: rifampin, rifapentine
- Glucocorticoid (systemic): dexamethasone (more than a single-dose)
- Herbal Products: St John's wort (Hypericum perforatum)
- Proton Pump Inhibitors: e.g., dexlansoprazole, esomeprazole, lansoprazole, omeprazole, pantoprazole, rabeprazole
-
5 WARNINGS AND PRECAUTIONS
5.1 Severe Acute Exacerbation of Hepatitis B in Patients Coinfected with HIV-1 and HBV
Test all patients with HIV-1 for the presence of chronic hepatitis B virus (HBV) before or when initiating antiretroviral therapy [see Dosage and Administration (2.1)].
Severe acute exacerbations of hepatitis B (e.g., liver decompensation and liver failure) have been reported in patients who are coinfected with HBV and HIV-1 and have discontinued products containing FTC and/or TDF, two of the components of COMPLERA. Patients coinfected with HIV-1 and HBV who discontinue COMPLERA should be closely monitored with both clinical and laboratory follow-up for at least several months after stopping treatment with COMPLERA. If appropriate, initiation of anti-hepatitis B therapy may be warranted, especially in patients with advanced liver disease or cirrhosis, since posttreatment exacerbation of hepatitis may lead to hepatic decompensation and liver failure.
5.2 Skin and Hypersensitivity Reactions
Severe skin and hypersensitivity reactions have been reported during the postmarketing experience, including cases of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) with RPV-containing regimens. While some skin reactions were accompanied by constitutional symptoms such as fever, other skin reactions were associated with organ dysfunctions, including elevations in hepatic serum biochemistries. During the Phase 3 clinical trials, treatment-related rashes with at least Grade 2 severity were reported in 1% of subjects receiving RPV plus FTC/TDF. Overall, most rashes were Grade 1 or 2 and occurred in the first four to six weeks of therapy [see Adverse Reactions (6.1 and 6.2)].
Discontinue COMPLERA immediately if signs or symptoms of severe skin or hypersensitivity reactions develop, including but not limited to, severe rash or rash accompanied by fever, blisters, mucosal involvement, conjunctivitis, facial edema, angioedema, hepatitis, or eosinophilia. Clinical status including laboratory parameters should be monitored and appropriate therapy should be initiated.
5.3 Hepatotoxicity
Hepatic adverse events have been reported in patients receiving an RPV-containing regimen. Patients with underlying hepatitis B or C virus infection, or marked elevations in liver-associated tests prior to treatment, may be at increased risk for worsening or development of liver-associated test elevations with use of COMPLERA. A few cases of hepatic toxicity have been reported in adult patients receiving an RPV-containing regimen who had no pre-existing hepatic disease or other identifiable risk factors. Appropriate laboratory testing prior to initiating therapy and monitoring for hepatotoxicity during therapy with COMPLERA is recommended in patients with underlying hepatic disease such as hepatitis B or C, or in patients with marked elevations in liver-associated tests prior to treatment initiation. Liver-associated test monitoring should also be considered for patients without pre-existing hepatic dysfunction or other risk factors.
5.4 Depressive Disorders
The adverse reaction depressive disorders (depressed mood, depression, dysphoria, major depression, mood altered, negative thoughts, suicide attempt, suicidal ideation) has been reported with RPV. Patients with severe depressive symptoms should seek immediate medical evaluation to assess the possibility that the symptoms are related to COMPLERA, and if so, to determine whether the risks of continued therapy outweigh the benefits.
During the Phase 3 trials in adults (N=1368) through 96 weeks, the incidence of depressive disorders (regardless of causality, severity) reported among RPV (n=686) or efavirenz (EFV, n=682) was 9% and 8%, respectively. Most events were mild or moderate in severity. The incidence of Grades 3 and 4 depressive disorders (regardless of causality) was 1% for both RPV and EFV. The incidence of discontinuation due to depressive disorders among RPV or EFV was 1% in each arm. Suicidal ideation was reported in 4 subjects in each arm while suicide attempt was reported in 2 subjects in the RPV arm.
During the Phase 2 trial in pediatric subjects 12 to less than 18 years of age (N=36) receiving RPV through 48 weeks, the incidence of depressive disorders (regardless of causality, severity) was 19.4% (7/36). Most events were mild or moderate in severity. The incidence of Grade 3 and 4 depressive disorders (regardless of causality) was 5.6% (2/36). None of the subjects discontinued due to depressive disorders. Suicidal ideation and suicide attempt were reported in 1 subject.
5.5 New Onset or Worsening Renal Impairment
Renal impairment, including cases of acute renal failure and Fanconi syndrome (renal tubular injury with severe hypophosphatemia), has been reported with the use of TDF [see Adverse Reactions (6.2)].
Prior to initiation of COMPLERA, and during treatment with COMPLERA, on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose, and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorus.
COMPLERA should be avoided with concurrent or recent use of a nephrotoxic agent (e.g., high-dose or multiple nonsteroidal anti-inflammatory drugs [NSAIDs]) [see Drug Interactions (7.4)]. Cases of acute renal failure after initiation of high-dose or multiple NSAIDs have been reported in HIV-infected patients with risk factors for renal dysfunction who appeared stable on TDF. Some patients required hospitalization and renal replacement therapy. Alternatives to NSAIDs should be considered, if needed, in patients at risk for renal dysfunction.
Persistent or worsening bone pain, pain in extremities, fractures, and/or muscular pain or weakness may be manifestations of proximal renal tubulopathy and should prompt an evaluation of renal function in at-risk patients.
Emtricitabine and TDF are principally eliminated by the kidney; however, RPV is not. Since COMPLERA is a combination product and the dose of the individual components cannot be altered, COMPLERA is not recommended in patients with estimated creatinine clearance below 50 mL per minute [see Use in Specific Populations (8.6)].
5.6 Bone Loss and Mineralization Defects
Bone Mineral Density
In clinical trials in HIV-1-infected adults, TDF, a component of COMPLERA, was associated with slightly greater decreases in bone mineral density (BMD) and increases in biochemical markers of bone metabolism, suggesting increased bone turnover relative to comparators. Serum parathyroid hormone levels and 1,25 Vitamin D levels were also higher in subjects receiving TDF.
Clinical trials evaluating TDF in pediatric and adolescent subjects were conducted. Under normal circumstances, BMD increases rapidly in pediatric patients. In HIV-1-infected subjects aged 2 years to less than 18 years, bone effects were similar to those observed in adult subjects and suggest increased bone turnover. Total body BMD gain was less in the TDF-treated HIV-1-infected pediatric subjects as compared to the control groups. Similar trends were observed in chronic hepatitis B-infected adolescent subjects aged 12 years to less than 18 years. In all pediatric trials, skeletal growth (height) appeared to be unaffected.
The effects of TDF-associated changes in BMD and biochemical markers on long-term bone health and future fracture risk are unknown. Assessment of BMD should be considered for adult and pediatric patients who have a history of pathologic bone fracture or other risk factors for osteoporosis or bone loss. Although the effect of supplementation with calcium and Vitamin D was not studied, such supplementation may be beneficial for all patients. If bone abnormalities are suspected, then appropriate consultation should be obtained.
Mineralization Defects
Cases of osteomalacia associated with proximal renal tubulopathy, manifested as bone pain or pain in extremities and which may contribute to fractures, have been reported in association with the use of TDF [see Adverse Reactions (6.2)]. Arthralgias and muscle pain or weakness have also been reported in cases of proximal renal tubulopathy. Hypophosphatemia and osteomalacia secondary to proximal renal tubulopathy should be considered in patients at risk of renal dysfunction who present with persistent or worsening bone or muscle symptoms while receiving products containing TDF [See Warnings and Precautions (5.5)].
5.7 Risk of Adverse Reactions or Loss of Virologic Response Due to Drug Interactions
The concomitant use of COMPLERA and other drugs may result in potentially significant drug interactions, some of which may lead to [see Dosage and Administration (2.5), Contraindications (4), and Drug Interactions (7)]:
- Loss of therapeutic effect of COMPLERA and possible development of resistance due to reduced exposure to RPV.
- Possible clinically significant adverse reaction from greater exposures of components of COMPLERA.
In healthy subjects, 75 mg once daily and 300 mg once daily doses of RPV (3 times and 12 times the dose in COMPLERA) have been shown to prolong the QTc interval of the electrocardiogram. Consider alternatives to COMPLERA when coadministered with a drug that is known to have a risk of Torsade de Pointes [see Drug Interactions (7) and Clinical Pharmacology (12.2)].
See Table 4 for steps to prevent or manage these possible and known significant drug interactions, including dosing recommendations. Consider the potential for drug interactions prior to and during COMPLERA therapy and review concomitant medications during COMPLERA therapy.
5.8 Lactic Acidosis/Severe Hepatomegaly with Steatosis
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs, including TDF and FTC, components of COMPLERA, alone or in combination with other antiretrovirals. Treatment with COMPLERA should be suspended in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
5.9 Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including the components of COMPLERA. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia [PCP], or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves' disease, polymyositis, Guillain-Barré syndrome, and autoimmune hepatitis) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable and can occur many months after initiation of treatment.
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in other sections of the labeling:
- Severe Acute Exacerbations of Hepatitis B in Patients Coinfected with HIV-1 and HBV[see Warnings and Precautions (5.1)].
- Skin and Hypersensitivity Reactions [see Warnings and Precautions (5.2)].
- Hepatotoxicity [see Warnings and Precautions (5.3)].
- Depressive Disorders [see Warnings and Precautions (5.4)].
- New Onset or Worsening Renal Impairment [see Warnings and Precautions (5.5)].
- Bone Loss and Mineralization Defects [see Warnings and Precautions (5.6)].
- Lactic Acidosis/Severe Hepatomegaly with Steatosis [see Warnings and Precautions (5.8)].
- Immune Reconstitution Syndrome [see Warnings and Precautions (5.9)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions from Clinical Trials Experience in Adult Subjects
In HIV-1-Infected Adult Subjects With No Antiretroviral Treatment History
Studies C209 and C215
The safety assessment of RPV, used in combination with other antiretroviral drugs, is based on the Week 96 pooled data from 1368 subjects in the Phase 3 trials TMC278-C209 (ECHO) and TMC278-C215 (THRIVE) in antiretroviral treatment-naïve HIV-1-infected adult subjects. A total of 686 subjects received RPV in combination with other antiretroviral drugs as background regimen; most (N=550) received FTC/TDF as background regimen. The number of subjects randomized to the control arm EFV was 682, of which 546 received FTC/TDF as background regimen [see Clinical Studies (14)]. The median duration of exposure for subjects in either treatment arm was 104 weeks.
Adverse reactions observed at Week 96 in subjects who received RPV or EFV + FTC/TDF as background regimen are shown in Table 1. No new types of adverse reactions were identified between Week 48 and Week 96. The adverse reactions observed in this subset of subjects were generally consistent with those seen for the overall patient population participating in these studies (refer to the prescribing information for Edurant).
The proportion of subjects who discontinued treatment with RPV or EFV + FTC/TDF due to adverse reactions, regardless of severity, was 2% and 5%, respectively. The most common adverse reactions leading to discontinuation were psychiatric disorders: 9 (1.6%) subjects in the RPV + FTC/TDF arm and 12 (2.2%) subjects in the EFV + FTC/TDF arm. Rash led to discontinuation in 1 (0.2%) subject in the RPV + FTC/TDF arm and 10 (1.8%) subjects in the EFV + FTC/TDF arm.
Common Adverse Reactions: Clinical adverse reactions to RPV or EFV of at least moderate intensity (≥Grade 2) reported in at least 2% of adult subjects are shown in Table 1.
Table 1 Selected Adverse Reactions* (Grades 2–4) Reported in ≥2% of Adult Subjects Receiving RPV or EFV in Combination with FTC/TDF in Studies C209 and C215 (Week 96 Analysis) Preferred Term RPV + FTC/TDF EFV + FTC/TDF N=550 N=546 - * Frequencies of adverse reactions are based on all Grades 2–4 treatment-emergent adverse events assessed to be related to study drug.
- † Includes adverse reactions reported as depressed mood, depression, dysphoria, major depression, mood altered, negative thoughts, suicide attempt, suicide ideation.
Depressive disorders† 2% 2% Headache 2% 2% Insomnia 2% 2% Abnormal dreams 1% 3% Dizziness 1% 7% Nausea 1% 2% Rash 1% 5% Rilpivirine: Adverse reactions of at least moderate intensity (≥Grade 2) that occurred in less than 2% of subjects treated with RPV plus any of the allowed background regimens (N=686) in clinical studies C209 and C215 include (grouped by Body System): vomiting, diarrhea, abdominal discomfort, abdominal pain, fatigue, cholecystitis, cholelithiasis, decreased appetite, somnolence, sleep disorders, anxiety, glomerulonephritis membranous, glomerulonephritis mesangioproliferative, and nephrolithiasis.
In Virologically Suppressed HIV-1-Infected Adult Subjects
No new adverse reactions to COMPLERA were identified in stable, virologically suppressed subjects switching to COMPLERA from a regimen containing a ritonavir-boosted protease inhibitor; however, the frequency of adverse reactions increased by 20% (Study 106) after switching to COMPLERA.
Emtricitabine and Tenofovir DF: The most common adverse reactions that occurred in at least 10% of HIV-1-infected treatment-naïve adult subjects in a Phase 3 clinical trial of FTC and TDF in combination with another antiretroviral agent were diarrhea, nausea, fatigue, headache, dizziness, depression, insomnia, abnormal dreams, and rash. Adverse reactions that occurred in at least 5% of treatment-experienced or treatment-naïve subjects receiving FTC or TDF with other antiretroviral agents in clinical trials included abdominal pain, dyspepsia, vomiting, fever, pain, nasopharyngitis, pneumonia, sinusitis, upper respiratory tract infection, arthralgia, back pain, myalgia, paresthesia, peripheral neuropathy (including peripheral neuritis and neuropathy), anxiety, increased cough, and rhinitis.
Skin discoloration has been reported with higher frequency among FTC-treated subjects; it was manifested by hyperpigmentation on the palms and/or soles and was generally mild and asymptomatic. The mechanism and clinical significance are unknown.
Laboratory Abnormalities in Adult Subjects
The percentage of subjects treated with RPV + FTC/TDF or EFV + FTC/TDF in studies C209 and C215 with selected laboratory abnormalities (Grades 1–4), representing worst-grade toxicity, is presented in Table 2.
Table 2 Selected Laboratory Abnormalities (Grades 1–4) Reported in Adult Subjects Who Received RPV or EFV in Combination with FTC/TDF in Studies C209 and C215 (Week 96 Analysis) Laboratory Parameter Abnormality DAIDS Toxicity Range RPV + FTC/TDF EFV + FTC/TDF N=550 N=546 N=number of subjects per treatment group
ULN=Upper limit of normal value.
Note: Percentages were calculated versus the number of subjects in ITT population with FTC + TDF as background regimen.BIOCHEMISTRY Increased Creatinine Grade 1 1.1–1.3 × ULN 6% 1% Grade 2 >1.3–1.8 × ULN 1% 1% Grade 3 >1.8–3.4 × ULN <1% 0 Grade 4 >3.4 × ULN 0 <1% Increased AST Grade 1 1.25–2.5 × ULN 16% 19% Grade 2 >2.5–5.0 × ULN 4% 7% Grade 3 >5.0–10.0 × ULN 2% 3% Grade 4 >10.0 × ULN 1% 1% Increased ALT Grade 1 1.25–2.5 × ULN 19% 22% Grade 2 >2.5–5.0 × ULN 5% 7% Grade 3 >5.0–10.0 × ULN 1% 2% Grade 4 >10.0 × ULN 1% 1% Increased Total Bilirubin Grade 1 1.1–1.5 × ULN 6% <1% Grade 2 >1.5–2.5 × ULN 3% 1% Grade 3 >2.5–5.0 × ULN 1% <1% Increased Total Cholesterol (fasted) Grade 1 200–239 mg/dL 14% 31% Grade 2 240–300 mg/dL 6% 18% Grade 3 >300 mg/dL <1% 2% Increased LDL Cholesterol (fasted) Grade 1 130–159 mg/dL 13% 28% Grade 2 160–190 mg/dL 5% 13% Grade 3 >190 mg/dL 1% 4% Increased Triglycerides (fasted) Grade 2 500–750 mg/dL 1% 2% Grade 3 751–1200 mg/dL 1% 2% Grade 4 >1200 mg/dL 0 1% Emtricitabine or Tenofovir DF: The following Grade 3 or 4 laboratory abnormalities have been previously reported in subjects treated with FTC or TDF with other antiretroviral agents in other clinical trials: increased pancreatic amylase (>2.0 × ULN), increased serum amylase (>175 U/L), increased lipase (>3.0 × ULN), increased alkaline phosphatase (>550 U/L), increased or decreased serum glucose (<40 or >250 mg/dL), increased glycosuria (≥3+), increased creatine kinase (M: >990 U/L; F: >845 U/L), decreased neutrophils (<750/mm3), and increased hematuria (>75 RBC/HPF).
Adrenal Function: In the pooled Phase 3 trials of C209 and C215, in subjects treated with RPV plus any of the allowed background regimens (N=686), at Week 96 there was an overall mean change from baseline in basal cortisol of –0.69 (−1.12, 0.27) micrograms/dL in the RPV group, and of −0.02 (−0.48, 0.44) micrograms/dL in the EFV group.
In the RPV group, 43/588 (7.3%) of subjects with a normal 250 micrograms ACTH stimulation test at baseline developed an abnormal 250 micrograms ACTH stimulation test (peak cortisol level <18.1 micrograms/dL) during the trial compared to 18/561 (3.2%) in the EFV group. Of the subjects who developed an abnormal 250 micrograms ACTH stimulation test during the trial, 14 subjects in the RPV group and 9 subjects in the EFV group had an abnormal 250 micrograms ACTH stimulation test at Week 96. Overall, there were no serious adverse events, deaths, or treatment discontinuations that could clearly be attributed to adrenal insufficiency. The clinical significance of the higher abnormal rate of 250 micrograms ACTH stimulation tests in the RPV group is not known.
Serum Creatinine: In the pooled Phase 3 trials of C209 and C215 in subjects treated with RPV plus any of the allowed background regimens (N=686), there was a small increase in serum creatinine over 96 weeks of treatment with RPV. Most of this increase occurred within the first 4 weeks of treatment, with a mean change of 0.1 mg/dL (range –0.3 to 0.6 mg/dL) observed through Week 96. In subjects who entered the trial with mild or moderate renal impairment, the serum creatinine increase observed was similar to that seen in subjects with normal renal function. These changes are not considered to be clinically relevant, and no subject discontinued treatment due to increases in serum creatinine. Creatinine increases were comparable by background N(t)RTIs.
Serum Lipids: Changes from baseline in total cholesterol, LDL-cholesterol, and triglycerides are presented in Table 3.
Table 3 Lipid Values Reported in Adult Subjects Receiving RPV or EFV in Combination with FTC/TDF in Studies C209 and C215* Pooled Data from the Week 96 Analysis of C209 and C215 Trials RPV + FTC/TDF
N=550EFV + FTC/TDF
N=546N Baseline Week 96 N Baseline Week 96 Mean Mean
(mg/dL)Mean
(mg/dL)Mean Change†
(mg/dL)Mean
(mg/dL)Mean
(mg/dL)Mean Change†
(mg/dL)N=number of subjects per treatment group - * Excludes subjects who received lipid lowering agents during the treatment period.
- † The change from baseline is the mean of within-patient changes from baseline for patients with both baseline and Week 96 values.
Total Cholesterol (fasted) 430 162 164 2 401 160 186 26 HDL-cholesterol (fasted) 429 42 45 4 399 40 50 11 LDL-cholesterol (fasted) 427 97 97 –1 397 96 110 14 Triglycerides (fasted) 430 123 109 –14 401 127 133 6 Adult Subjects Coinfected with Hepatitis B and/or Hepatitis C Virus: In adult subjects coinfected with hepatitis B or C virus receiving RPV in studies C209 and C215, the incidence of hepatic enzyme elevation was higher than in subjects receiving RPV who were not coinfected. The same increase was also observed in the EFV arm. The pharmacokinetic exposure of RPV in coinfected subjects was comparable to that in subjects without coinfection.
Adverse Reactions from Clinical Trials Experience in Pediatric Subjects
Emtricitabine: In addition to the adverse reactions reported in adults, anemia and hyperpigmentation were observed in 7% and 32%, respectively, of pediatric subjects (3 months to less than 18 years of age) who received treatment with FTC in the larger of two open-label, uncontrolled pediatric trials (N=116). For additional information, please consult the EMTRIVA® prescribing information.
Rilpivirine: The safety assessment is based on the Week 48 analysis of the single-arm, open-label Phase 2 trial, TMC278-C213, in which 36 antiretroviral treatment-naïve HIV-1-infected subjects 12 to less than 18 years of age and weighing at least 32 kg received RPV (25 mg once daily) in combination with other antiretroviral agents. The median duration of exposure for subjects was 63.5 weeks. No subjects discontinued treatment due to adverse reactions. No new adverse reactions were identified compared to those seen in adults.
Adverse reactions were reported in 19 pediatric subjects (52.8%). Most adverse reactions were Grade 1 or 2. The most common adverse reactions reported in at least 2 subjects (regardless of severity) include headache (19.4%), depression (19.4%), somnolence (13.9%), nausea (11.1%), dizziness (8.3%), abdominal pain (8.3%), vomiting (5.6%), and rash (5.6%).
Observed laboratory abnormalities were comparable to those in adults. For additional information, please consult the Edurant prescribing information.
Adrenal Function
In trial TMC278-C213, at Week 48, the overall mean change from baseline in basal cortisol showed an increase of 1.59 (0.24, 2.93) micrograms/dL.
Six of 30 (20%) subjects with a normal 250 micrograms ACTH stimulation test at baseline developed an abnormal 250 micrograms ACTH stimulation test (peak cortisol level <18.1 micrograms/dL) during the trial. Three of these subjects had an abnormal 250 micrograms ACTH stimulation test at Week 48. Overall, there were no serious adverse events, deaths, or treatment discontinuations that could clearly be attributed to adrenal insufficiency. The clinical significance of the abnormal 250 micrograms ACTH stimulation tests is not known.
Tenofovir DF: In a pediatric clinical trial conducted in subjects 12 to less than 18 years of age, the adverse reactions observed in pediatric subjects who received treatment with TDF were consistent with those observed in clinical trials of TDF in adults [see Warnings and Precautions (5.6)]. For additional information, including information on bone mineral density changes, please consult the VIREAD® prescribing information.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postmarketing experience in patients receiving RPV- or TDF-containing regimens. Because postmarketing reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
COMPLERA:
Metabolism and Nutrition Disorders
weight increasedSkin and Subcutaneous Tissue Disorders
severe skin and hypersensitivity reactions including DRESS (Drug Reaction with Eosinophilia and Systemic Symptoms)Emtricitabine:
No postmarketing adverse reactions have been identified for inclusion in this section.
Tenofovir DF:
Immune System Disorders
allergic reaction, including angioedemaMetabolism and Nutrition Disorders
lactic acidosis, hypokalemia, hypophosphatemiaRespiratory, Thoracic, and Mediastinal Disorders
dyspneaGastrointestinal Disorders
pancreatitis, increased amylase, abdominal painHepatobiliary Disorders
hepatic steatosis, hepatitis, increased liver enzymes (most commonly AST, ALT, gamma GT)Skin and Subcutaneous Tissue Disorders
rashMusculoskeletal and Connective Tissue Disorders
rhabdomyolysis, osteomalacia (manifested as bone pain and which may contribute to fractures), muscular weakness, myopathyRenal and Urinary Disorders
acute renal failure, renal failure, acute tubular necrosis, Fanconi syndrome, proximal renal tubulopathy, interstitial nephritis (including acute cases), nephrogenic diabetes insipidus, renal insufficiency, increased creatinine, proteinuria, polyuriaGeneral Disorders and Administration Site Conditions
astheniaThe following adverse reactions, listed under the body system headings above, may occur as a consequence of proximal renal tubulopathy: rhabdomyolysis, osteomalacia, hypokalemia, muscular weakness, myopathy, hypophosphatemia.
-
7 DRUG INTERACTIONS
7.1 Not Recommended with Other Antiretroviral Medications
Because COMPLERA is a complete regimen, coadministration with other antiretroviral medications for the treatment of HIV-1 infection is not recommended. Comprehensive information regarding potential drug-drug interactions with other antiretroviral medications is not provided.
This section describes clinically relevant drug interactions with COMPLERA. Drug interaction studies were conducted with the components of COMPLERA (FTC, RPV, and TDF as single agents) or with COMPLERA as a combination product [see Dosage and Administration (2), Contraindications (4), and Clinical Pharmacology (12.3)].
7.2 Drugs Inducing or Inhibiting CYP3A Enzymes
Rilpivirine is primarily metabolized by cytochrome P450 (CYP) 3A, and drugs that induce or inhibit CYP3A may thus affect the clearance of RPV [see Contraindications (4), Warnings and Precautions (5.7), and Clinical Pharmacology (12.3)]. Coadministration of RPV and drugs that induce CYP3A may result in decreased plasma concentrations of RPV and loss of virologic response and possible resistance to RPV or to the class of NNRTIs. Coadministration of RPV and drugs that inhibit CYP3A may result in increased plasma concentrations of RPV.
7.3 Drugs Increasing Gastric pH
Coadministration of RPV with drugs that increase gastric pH may decrease plasma concentrations of RPV and loss of virologic response and possible resistance to RPV or to the class of NNRTIs. Use of RPV with proton pump inhibitors is contraindicated and use of RPV with H2-receptor antagonists requires staggered administration [see Contraindications (4) and Clinical Pharmacology (12.3)].
7.4 Drugs Affecting Renal Function
Because FTC and tenofovir are primarily eliminated by the kidneys through a combination of glomerular filtration and active tubular secretion, coadministration of COMPLERA with drugs that reduce renal function or compete for active tubular secretion may increase serum concentrations of FTC, tenofovir, and/or other renally eliminated drugs. Some examples of drugs that are eliminated by active tubular secretion include, but are not limited to, acyclovir, adefovir dipivoxil, cidofovir, ganciclovir, valacyclovir, valganciclovir, aminoglycosides (e.g., gentamicin), and high-dose or multiple NSAIDs [see Warnings and Precautions (5.5)].
7.5 QT Prolonging Drugs
There is limited information available on the potential for a pharmacodynamic interaction between RPV and drugs that prolong the QTc interval of the electrocardiogram. In a study of healthy subjects, 75 mg once daily and 300 mg once daily doses of RPV (3 times and 12 times the dose in COMPLERA) have been shown to prolong the QTc interval of the electrocardiogram [see Warnings and Precautions (5.7) and Clinical Pharmacology (12.2)]. Consider alternatives to COMPLERA when coadministered with a drug with a known risk of Torsade de Pointes.
7.6 Significant Drug Interactions
Important drug interaction information for COMPLERA is summarized in Table 4. The drug interactions described are based on studies conducted with FTC, RPV, or TDF as individual medications or with COMPLERA as a combination product, or are potential drug interactions [see Clinical Pharmacology (12.3), Tables 9–14]. For list of contraindicated drugs, [see Contraindications (4)].
Table 4 Significant* Drug Interactions Concomitant Drug Class: Drug Name Effect on Concentration† Clinical Comment - * This table is not all inclusive.
- † Increase = ↑; Decrease = ↓; No Effect = ↔
- ‡ The interaction was evaluated in a clinical study. All other drug-drug interactions shown are predicted.
- § This interaction study has been performed with a dose higher than the recommended dose for RPV assessing the maximal effect on the coadministered drug. The dosing recommendation is applicable to the recommended dose of RPV 25 mg once daily.
Antacids:
antacids
(e.g., aluminum, magnesium hydroxide, or calcium carbonate)↔ RPV
(antacids taken at least 2 hours before or at least 4 hours after RPV)
↓ RPV (concomitant intake)Administer antacids at least 2 hours before or at least 4 hours after COMPLERA. Anticonvulsants:
carbamazepine
oxcarbazepine
phenobarbital
phenytoin↓ RPV Coadministration is contraindicated due to potential for loss of virologic response and development of resistance. Antimycobacterials:
rifampin
rifapentine↓ RPV Coadministration is contraindicated due to potential for loss of virologic response and development of resistance. rifabutin ↓ RPV‡ If COMPLERA is coadministered with rifabutin, an additional 25 mg tablet of RPV (Edurant) once per day is recommended to be taken concomitantly with COMPLERA and with a meal for the duration of rifabutin coadministration. Azole Antifungal Agents:
fluconazole
itraconazole
ketoconazole
posaconazole
voriconazole↑ RPV‡,§
↓ ketoconazole‡,§No dose adjustment is required when COMPLERA is coadministered with azole antifungal agents. Clinically monitor for breakthrough fungal infections when azole antifungals are coadministered with COMPLERA. Glucocorticoid (systemic):
dexamethasone
(more than a single-dose treatment)↓ RPV Coadministration is contraindicated due to potential for loss of virologic response and development of resistance. Hepatitis C Antiviral Agents:
ledipasvir/sofosbuvir
sofosbuvir/velpatasvir
sofosbuvir/velpatasvir/voxilaprevir↑ tenofovir‡ Patients receiving COMPLERA concomitantly with HARVONI® (ledipasvir/sofosbuvir), EPCLUSA® (sofosbuvir/velpatasvir), or VOSEVI® (sofosbuvir/velpatasvir/voxilaprevir) should be monitored for adverse reactions associated with TDF. H2-Receptor Antagonists:
cimetidine
famotidine
nizatidine
ranitidine↔ RPV‡,§
(famotidine taken 12 hours before RPV or 4 hours after RPV)
↓ RPV‡,§
(famotidine taken 2 hours before RPV)Administer H2-receptor antagonists at least 12 hours before or at least 4 hours after COMPLERA. Herbal Products:
St John's wort
(Hypericum perforatum)↓ RPV Coadministration is contraindicated due to potential for loss of virologic response and development of resistance. Macrolide or Ketolide Antibiotics:
clarithromycin
erythromycin
telithromycin↑ RPV
↔ clarithromycin
↔ erythromycin
↔ telithromycinWhere possible, alternatives such as azithromycin should be considered. Narcotic Analgesics:
methadone↓ R(–) methadone‡
↓ S(+) methadone‡
↔ RPV‡
↔ methadone‡ (when used with tenofovir)No dose adjustments are required when initiating coadministration of methadone with COMPLERA. However, clinical monitoring is recommended as methadone maintenance therapy may need to be adjusted in some patients. Proton Pump Inhibitors:
e.g., dexlansoprazole
esomeprazole
lansoprazole
omeprazole
pantoprazole
rabeprazole↓ RPV Coadministration is contraindicated due to potential for loss of virologic response and development of resistance. 7.7 Drugs with No Observed Interactions with COMPLERA
No clinically significant drug interactions have been observed between FTC and the following medications: famciclovir, ledipasvir/sofosbuvir, sofosbuvir/velpatasvir, sofosbuvir/velpatasvir/voxilaprevir, or TDF.
No clinically significant drug interactions have been observed between TDF and the following medications: entecavir, methadone, oral contraceptives, ribavirin, sofosbuvir, or tacrolimus in studies conducted in healthy subjects.
No clinically significant drug interactions have been observed between RPV and the following medications: acetaminophen, atorvastatin, chlorzoxazone, ethinyl estradiol, ledipasvir/sofosbuvir, norethindrone, sildenafil, simeprevir, sofosbuvir, sofosbuvir/velpatasvir, sofosbuvir/velpatasvir/voxilaprevir, or TDF. RPV did not have a clinically significant effect on the pharmacokinetics of digoxin or metformin.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in individuals exposed to COMPLERA during pregnancy. Healthcare providers are encouraged to register patients by calling the Antiretroviral Pregnancy Registry (APR) at 1-800-258-4263.
Risk Summary
Available data from the APR show no increase in the overall risk of major birth defects with first trimester exposure for emtricitabine (FTC), rilpivirine (RPV), or tenofovir (TDF) compared with the background rate for major birth defects of 2.7% in a U.S. reference population of the Metropolitan Atlanta Congenital Defects Program (MACDP) (see Data). In a clinical trial, total rilpivirine exposures were generally lower during pregnancy compared to the postpartum period [see Clinical Pharmacology (12.3)]. The rate of miscarriage for individual drugs is not reported in the APR. The estimated background rate of miscarriage in clinically recognized pregnancies in the U.S. general population is 15–20%.
Based on the experience of HIV-1-infected pregnant individuals who completed a clinical trial through the postpartum period with an RPV-based regimen, no dose adjustments are required for pregnant patients who are already on a stable RPV-containing regimen prior to pregnancy and who are virologically suppressed (HIV-1 RNA less than 50 copies per mL). Lower exposures of RPV were observed during pregnancy, therefore viral load should be monitored closely [see Data and Clinical Pharmacology (12.3)].
In animal studies, no adverse developmental effects were observed when the components of COMPLERA were administered separately during the period of organogenesis at exposures up to 60 and 120 times (mice and rabbits, respectively, FTC) and 15 and 70 times (rats and rabbits, respectively; RPV) the exposure of these components in COMPLERA and at 14 and 19 times (rats and rabbits, respectively) the human dose of TDF based on body surface area comparisons (see Data). Likewise, no adverse developmental effects were seen when FTC was administered to mice and RPV was administered to rats through lactation at exposures up to approximately 60 and 63 times, respectively, the exposure at the recommended daily dose of these components in COMPLERA. No adverse effects were observed in the offspring of rats when TDF was administered through lactation at tenofovir exposures of approximately 14 times the exposure at the recommended daily dosage of COMPLERA.
Data
Human Data
Prospective reports from the APR of overall major birth defects in pregnancies exposed to drug components of COMPLERA are compared with a U.S. background major birth defect rate. Methodological limitations of the APR include the use of MACDP as the external comparator group. Limitations of using an external comparator include differences in methodology and populations, as well as confounding due to the underlying disease.
Emtricitabine: Based on prospective reports to the APR of exposures to FTC-containing regimens during pregnancy resulting in live births (including over 2,750 exposed in the first trimester and over 1,200 exposed in the second/third trimester), there was no increase in overall major birth defects with FTC compared with the background birth defect rate of 2.7% in the U.S. reference population of the MACDP. The prevalence of major birth defects in live births was 2.4% (95% CI: 1.9% to 3.1%) with first trimester exposure to FTC-containing regimens and 2.3% (95% CI: 1.5% to 3.3%) with the second/third trimester exposure to FTC-containing regimens.
Rilpivirine: RPV in combination with a background regimen was evaluated in a clinical trial of 19 HIV-1 infected pregnant subjects on an RPV-based regimen during the second and third trimesters and postpartum. Each of the subjects were on an RPV-based regimen at the time of enrollment. Twelve subjects completed the trial through the postpartum period (6–12 weeks after delivery) and pregnancy outcomes are missing for six subjects. The exposure (C0h and AUC) of total RPV was approximately 30 to 40% lower during pregnancy compared with postpartum (6 to 12 weeks). The protein binding of RPV was similar (>99%) during second trimester, third trimester, and postpartum period [see Clinical Pharmacology (12.3)]. One subject discontinued the trial following fetal death at 25 weeks gestation due to suspected premature rupture of membranes. Among the 12 subjects who were virologically suppressed at baseline (less than 50 copies/mL), virologic response was preserved in 10 subjects (83.3%) through the third trimester visit and in 9 subjects (75%) through the 6–12 week postpartum visit. Virologic outcomes during the third trimester visit were missing for two subjects who were withdrawn (one subject was nonadherent to the study drug and one subject withdrew consent). Among the 10 infants with available HIV test results, all were negative for HIV-1 at the time of delivery and up to 16 weeks postpartum (all 10 infants received prophylactic treatment with zidovudine). RPV was well tolerated during pregnancy and postpartum. There were no new safety findings compared with the known safety profile of RPV in HIV–1-infected adults.
Based on prospective reports to the APR of exposures to RPV-containing regimens during pregnancy (including over 290 exposed during first trimester and over 160 exposed in the second/third trimester), there was no significant increase in overall risk of major birth defects with RPV compared to the background birth defect rate of 2.7% in the U.S. reference population of the MACDP. The prevalence of major birth defects in live births was 1.0% (95% CI: 0.2% to 2.9%) and 1.2% (95% CI: 0.2% to 4.4%) following first and second/third trimester exposure, respectively, to RPV-containing regimens.
Tenofovir DF: Based on prospective reports to the APR of exposures to TDF-containing regimens during pregnancy resulting in live births (including over 3,500 exposed in the first trimester and over 1,500 exposed in the second/third trimester), there was no increase in overall risk of major birth defects compared with the background birth defect rate of 2.7% in the U.S. reference population of the MACDP. The prevalence of major birth defects in live births was 2.3% (95% CI: 1.8% to 2.9%) with first trimester exposure to TDF-containing regimens, and 2.2% (95% CI: 1.6% to 3.1%) with the second/third trimester exposure to TDF-containing regimens.
Animal Data
Emtricitabine: FTC was administered orally to pregnant mice (at 0, 250, 500, or 1,000 mg/kg/day), and rabbits (at 0, 100, 300, or 1,000 mg/kg/day) through organogenesis (on gestation days 6 through 15, and 7 through 19, respectively). No significant toxicological effects were observed in embryo-fetal toxicity studies performed with FTC in mice at exposures (AUC) approximately 60 times higher and in rabbits at approximately 120 times higher than human exposures at the recommended daily dose. In a pre/postnatal development study in mice, FTC was administered orally at doses up to 1,000 mg/kg/day; no significant adverse effects directly related to drug were observed in the offspring exposed daily from before birth (in utero) through sexual maturity at daily exposures (AUC) of approximately 60 times higher than human exposures at the recommended daily dose.
Rilpivirine: RPV was administered orally to pregnant rats (40, 120, or 400 mg/kg/day) and rabbits (5, 10, or 20 mg/kg/day) through organogenesis (on gestation days 6 through 17, and 6 through 19, respectively). No significant toxicological effects were observed in embryo-fetal toxicity studies performed with RPV in rats and rabbits at exposures 15 (rats) and 70 (rabbits) times higher than the exposure in humans at the recommended dose of 25 mg once daily. In a pre/postnatal development study with RPV, where rats were administered up to 400 mg/kg/day through lactation, no significant adverse effects directly related to drug were noted in the offspring.
Tenofovir DF: TDF was administered orally to pregnant rats (at 0, 50, 150, or 450 mg/kg/day) and rabbits (at 0, 30, 100, or 300 mg/kg/day) through organogenesis (on gestation days 7 through 17, and 6 through 18, respectively). No significant toxicological effects were observed in embryo-fetal toxicity studies performed with TDF in rats at doses up to 14 times the human dose based on body surface area comparisons and in rabbits at doses up to 19 times the human dose based on body surface area comparisons. In a pre/postnatal development study in rats, TDF was administered orally through lactation at doses up to 600 mg/kg/day; no adverse effects were observed in the offspring at tenofovir exposures of approximately 2.7 times higher than human exposures at the recommended daily dose of COMPLERA.
8.2 Lactation
Risk Summary
The Centers for Disease Control and Prevention recommend that HIV infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV.
Based on published data, FTC and tenofovir have been shown to be present in human milk. There are no data on the presence of RPV in human milk. RPV has been shown to be present in rat milk (see Data).
It is not known if the components of COMPLERA affect milk production or have effects on the breastfed child. Because of the potential for: (1) HIV transmission (in HIV-negative infants); (2) developing viral resistance (in HIV-positive infants); and (3) adverse reactions in a breastfed infant similar to those seen in adults, instruct mothers not to breastfeed if they are receiving COMPLERA.
8.4 Pediatric Use
The safety and effectiveness of COMPLERA as a complete regimen for the treatment of HIV-1 infection was established in pediatric subjects 12 years of age and older with body weight greater than or equal to 35 kg [see Dosage and Administration (2.2)]. Use of COMPLERA in this age group weighing at least 35 kg is supported by adequate and well-controlled studies of RPV+FTC+TDF in adults with HIV-1 infection as well as data from pediatric studies of the individual components of COMPLERA (RPV, FTC, and TDF) [see Clinical Pharmacology (12.3), and Clinical Studies (14.2)].
COMPLERA should only be administered to pediatric patients with a body weight greater than or equal to 35 kg. Because COMPLERA is a fixed-dose combination tablet, the dose of COMPLERA cannot be adjusted for patients of lower weight. Safety and effectiveness for COMPLERA have not been established in pediatric patients weighing less than 35 kg [see Adverse Reactions (6.1) and Clinical Pharmacology (12.3)].
8.5 Geriatric Use
Clinical studies of FTC, RPV, or TDF did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for elderly patients should be cautious, keeping in mind the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy [see Clinical Pharmacology (12.3)].
8.6 Renal Impairment
Because COMPLERA is a fixed-dose combination, and cannot be dose adjusted, it is not recommended in patients with moderate, severe, or end-stage renal impairment (estimated creatinine clearance below 50 mL per minute) or that require dialysis [see Warnings and Precautions (5.5) and Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No dose adjustment of COMPLERA is required in patients with mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment. COMPLERA has not been studied in patients with severe hepatic impairment (Child-Pugh Class C) [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
If overdose occurs the patient must be monitored for evidence of toxicity. Treatment of overdose with COMPLERA consists of general supportive measures, including monitoring of vital signs and ECG (QT interval) as well as observation of the clinical status of the patient.
Emtricitabine: Hemodialysis treatment removes approximately 30% of the FTC dose over a 3-hour dialysis period starting within 1.5 hours of FTC dosing (blood flow rate of 400 mL per minute and a dialysate flow rate of 600 mL per minute). It is not known whether FTC can be removed by peritoneal dialysis.
-
11 DESCRIPTION
COMPLERA is a fixed-dose combination tablet containing FTC, rilpivirine hydrochloride, and TDF. Emtricitabine (FTC) is a synthetic nucleoside analog of cytidine. Rilpivirine (RPV) is a non-nucleoside reverse transcriptase inhibitor. Tenofovir disoproxil fumarate (TDF) is converted in vivo to tenofovir, an acyclic nucleoside phosphonate (nucleotide) analog of adenosine 5′-monophosphate.
COMPLERA tablets are for oral administration. Each tablet contains 200 mg of FTC, 27.5 mg of rilpivirine hydrochloride (equivalent to 25 mg of RPV), and 300 mg of TDF (equivalent to 245 mg of tenofovir disoproxil) as active ingredients. The tablets include the following inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polysorbate 20, povidone, pregelatinized starch. The tablets are film coated with a coating material containing FD&C Blue #2 aluminum lake, FD&C Yellow #6 aluminum lake, hypromellose, iron oxide red, lactose monohydrate, polyethylene glycol, titanium dioxide, triacetin.
Emtricitabine: The chemical name of FTC is 5-fluoro-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Emtricitabine is the (-) enantiomer of a thio analog of cytidine, which differs from other cytidine analogs in that it has a fluorine in the 5-position.
It has a molecular formula of C8H10FN3O3S and a molecular weight of 247.24. It has the following structural formula:
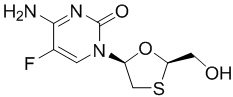
FTC is a white to off-white crystalline powder with a solubility of approximately 112 mg per mL in water at 25 °C.
Rilpivirine: RPV is available as the hydrochloride salt. The chemical name for rilpivirine hydrochloride is 4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethylphenyl]amino]-2-pyrimidinyl]amino]benzonitrile monohydrochloride. Its molecular formula is C22H18N6 ∙ HCl and its molecular weight is 402.88. Rilpivirine hydrochloride has the following structural formula:
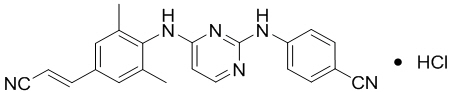
Rilpivirine hydrochloride is a white to almost white powder. Rilpivirine hydrochloride is practically insoluble in water over a wide pH range.
Tenofovir DF: TDF is a fumaric acid salt of the bis-isopropoxycarbonyloxymethyl ester derivative of tenofovir. The chemical name of TDF is 9-[(R)-2 [[bis[[(isopropoxycarbonyl)oxy]- methoxy]phosphinyl]methoxy]propyl]adenine fumarate (1:1). It has a molecular formula of C19H30N5O10P ∙ C4H4O4 and a molecular weight of 635.52. It has the following structural formula:
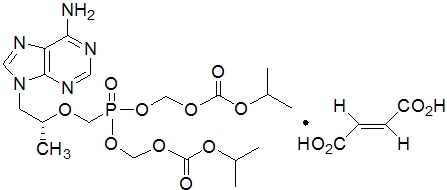
TDF is a white to off-white crystalline powder with a solubility of 13.4 mg per mL in water at 25 °C. All dosages are expressed in terms of TDF except where otherwise noted.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
COMPLERA is a fixed-dose combination of the antiretroviral drugs FTC, RPV, and TDF [see Microbiology (12.4)].
12.2 Pharmacodynamics
Effects on Electrocardiogram
The effect of RPV at the recommended dose of 25 mg once daily on the QTcF interval was evaluated in a randomized, placebo-, and active- (moxifloxacin 400 mg once daily) controlled crossover study in 60 healthy adults, with 13 measurements over 24 hours at steady state. The maximum mean time-matched (95% upper confidence bound) differences in QTcF interval from placebo after baseline correction was 2.0 (5.0) milliseconds (i.e., below the threshold of clinical concern).
When doses of 75 mg once daily and 300 mg once daily of RPV (3 times and 12 times the dose in COMPLERA) were studied in healthy adults, the maximum mean time-matched (95% upper confidence bound) differences in QTcF interval from placebo after baseline correction were 10.7 (15.3) and 23.3 (28.4) milliseconds, respectively. Steady-state administration of RPV 75 mg once daily and 300 mg once daily resulted in a mean steady-state Cmax approximately 2.6-fold and 6.7-fold, respectively, higher than the mean Cmax observed with the recommended 25 mg once daily dose of RPV [see Warnings and Precautions (5.7)].
12.3 Pharmacokinetics
COMPLERA: Under fed conditions (total calorie content of the meal was approximately 400 kcal with approximately 13 grams of fat), RPV, FTC, and tenofovir exposures were similar when comparing COMPLERA to EMTRIVA capsules (200 mg) plus Edurant tablets (25 mg) plus VIREAD tablets (300 mg) following single-dose administration to healthy subjects (N=34).
Single-dose administration of COMPLERA tablets to healthy subjects under fasted conditions provided approximately 25% higher exposure of RPV compared to administration of EMTRIVA capsules (200 mg) plus Edurant tablets (25 mg) plus VIREAD tablets (300 mg), while exposures of FTC and tenofovir were comparable (N=15).
Absorption, Distribution, Metabolism, and Excretion
The pharmacokinetic properties of the components of COMPLERA are provided in Table 5. The PK parameters of RPV, FTC, and tenofovir are provided in Table 6.
Table 5 Pharmacokinetic Properties of the Components of COMPLERA RPV FTC Tenofovir NC=Not Calculated - * Median
- † Oral bioavailability of tenofovir from VIREAD.
- ‡ Values refer to % change based on calculated geometric mean ratio [fed/fasted] in AUC.
COMPLERA light meal = 390 kcal, 12 g fat; COMPLERA standard meal = 540 kcal, 21 g fat. High fat meal not evaluated. Increase = ↑; Decrease = ↓; No Effect= ↔- § Mean ± SD
- ¶ t1/2 values refer to median terminal plasma half-life.
- # Dosing in mass balance studies: FTC (single dose administration of [14C] FTC after multiple dosing of FTC for 10 days); RPV (single dose administration of [14C] RPV); mass balance study not conducted for tenofovir.
Absorption Tmax (h) 4–5 1–2 1 % Fasted oral bioavailability* NC 93 25† Effect of a light meal (relative to fasting)‡ ↑9% ↔ ↑28% Effect of a standard meal (relative to fasting)‡ ↑16% ↔ ↑38% Distribution % Bound to human plasma proteins ~99 <4 <0.7 Source of protein binding data In vitro In vitro In vitro Metabolism Metabolism CYP3A Not significantly metabolized Elimination Major route of elimination Metabolism Glomerular filtration and active tubular secretion CLrenal§ (mL/min) NC 213±89 243±33 t1/2 (h)¶ 50 10 17 % Of dose excreted in urine# 6 86 70−80 % Of dose excreted in feces# 85 ~14 NC Table 6 Pharmacokinetic Parameters for RPV, FTC, and Tenofovir in HIV-Infected Adults Parameter
Mean ± SDRPV* FTC† Tenofovir‡ NA=Not Applicable; SD=Standard Deviation - * Population PK estimates of RPV 25 mg once daily in antiretroviral treatment-naïve HIV-1-infected adult subjects (pooled data from Phase 3 trials through Week 96; n=679)
- † Multiple-dose oral administration of FTC 200 mg to HIV-1-infected subjects (n=20)
- ‡ Single 300 mg dose of TDF to HIV-1-infected subjects in the fasted state
- § Data presented as steady state values
- ¶ AUC0–24h
Cmax (μg/mL) NA 1.80±0.72§ 0.30±0.09 AUCtau (μg∙hr/mL) 2.24±0.85§ 10.0±3.12§ 2.29±0.69¶ C0h (μg/mL) 0.08±0.04§ 0.09±0.07§ NA Specific Populations
Geriatric Patients
The pharmacokinetics of FTC, RPV, and tenofovir have not been fully evaluated in the elderly (65 years of age and older) [see Use in Specific Populations (8.5)].
Pediatric Patients
Pediatric trials have not been conducted using COMPLERA tablets. Pediatric information is based on trials conducted with the individual components of COMPLERA [see Use in Specific Populations (8.4)].
Emtricitabine: The pharmacokinetics of FTC at steady state were determined in 27 HIV-1-infected pediatric subjects 13 to 17 years of age receiving a daily dose of 6 mg/kg up to a maximum dose of 240 mg oral solution or a 200 mg capsule; 26 of 27 subjects in this age group received the 200 mg FTC capsule. Mean (± SD) Cmax and AUC were 2.7 ± 0.9 μg/mL and 12.6 ± 5.4 μg∙hr/mL, respectively. Exposures achieved in pediatric subjects 12 to less than 18 years of age were similar to those achieved in adults receiving a once daily dose of 200 mg.
Rilpivirine: The pharmacokinetics of RPV in antiretroviral treatment-naïve HIV-1- infected pediatric subjects 12 to less than 18 years of age receiving RPV 25 mg once daily were comparable to those in treatment-naïve HIV-1-infected adults receiving RPV 25 mg once daily (See Table 7). There was no clinically significant impact of body weight on RPV pharmacokinetics in pediatric subjects in trial C213 (33 to 93 kg).
Table 7 Population Pharmacokinetic Estimates of RPV 25 mg once daily in Antiretroviral Treatment-Naïve HIV-1-Infected Pediatric Subjects aged 12 to less than 18 years (Data from Phase 2 Trial through Week 48) Parameter RPV 25 mg once daily
N=34AUC24h (ng∙h/mL) Mean ± Standard Deviation 2424 ± 1024 Median (Range) 2269 (417−5166) C0h (ng/mL) Mean ± Standard Deviation 85 ± 40 Median (Range) 79 (7−202) Tenofovir DF: Steady-state pharmacokinetics of tenofovir were evaluated in 8 HIV-1-infected pediatric subjects (12 to less than 18 years). Mean (± SD) Cmax and AUCtau are 0.38 ± 0.13 μg/mL and 3.39 ± 1.22 μg∙hr/mL, respectively. Tenofovir exposure achieved in these pediatric subjects receiving oral daily doses of TDF 300 mg was similar to exposures achieved in adults receiving once-daily doses of TDF 300 mg.
Gender
No clinically relevant pharmacokinetic differences have been observed based on gender for FTC, RPV, and TDF.
Race
Emtricitabine: No pharmacokinetic differences due to race have been identified following the administration of FTC.
Patients with Renal Impairment
Emtricitabine and Tenofovir DF: The pharmacokinetics of FTC and TDF are altered in subjects with renal impairment. In subjects with creatinine clearance below 50 mL per minute or with end stage renal disease requiring dialysis, Cmax and AUC of FTC and tenofovir were increased [see Warnings and Precautions (5.5) and Use in Specific Populations (8.6)].
Rilpivirine: Population pharmacokinetic analysis indicated that RPV exposure was similar in HIV-1-infected subjects with mild renal impairment relative to HIV-1-infected subjects with normal renal function. There is limited or no information regarding the pharmacokinetics of RPV in patients with moderate or severe renal impairment or in patients with end-stage renal disease, and RPV concentrations may be increased due to alteration of drug absorption, distribution, and metabolism secondary to renal dysfunction [see Use in Specific Populations (8.6)].
Patients with Hepatic Impairment
Emtricitabine: The pharmacokinetics of FTC have not been studied in subjects with hepatic impairment; however, FTC is not significantly metabolized by liver enzymes, so the impact of liver impairment should be limited.
Rilpivirine: RPV is primarily metabolized and eliminated by the liver. In a study comparing 8 subjects with mild hepatic impairment (Child-Pugh score A) to 8 matched controls, and 8 subjects with moderate hepatic impairment (Child-Pugh score B) to 8 matched controls, the multiple dose exposure of RPV was 47% higher in subjects with mild hepatic impairment and 5% higher in subjects with moderate hepatic impairment. RPV has not been studied in subjects with severe hepatic impairment (Child-Pugh score C) [see Use in Specific Populations (8.7)].
Tenofovir DF: The pharmacokinetics of tenofovir following a 300 mg dose of TDF have been studied in non-HIV-infected subjects with moderate to severe hepatic impairment. There were no substantial alterations in tenofovir pharmacokinetics in subjects with hepatic impairment compared with unimpaired subjects.
Hepatitis B and/or Hepatitis C Virus Coinfection
The pharmacokinetics of FTC and TDF have not been fully evaluated in hepatitis B and/or C virus-coinfected patients. Population pharmacokinetic analysis indicated that hepatitis B and/or C virus coinfection had no clinically relevant effect on the exposure to RPV.
Pregnancy and Postpartum
The exposure (C0h and AUC24h) to total RPV after intake of RPV 25 mg once daily as part of an antiretroviral regimen was 30 to 40% lower during pregnancy (similar for the second and third trimester), compared with postpartum (see Table 8). However, the exposure during pregnancy was not significantly different from exposures obtained in Phase 3 trials of RPV-containing regimens. Based on the exposure-response relationship for RPV, this decrease is not considered clinically relevant in patients who are virologically suppressed. The protein binding of RPV was similar (>99%) during the second trimester, third trimester, and postpartum.
Table 8: Pharmacokinetic Results of Total RPV After Administration of RPV 25 mg Once Daily as Part of an Antiretroviral Regimen, During the 2nd Trimester of Pregnancy, the 3rd Trimester of Pregnancy and Postpartum Pharmacokinetics of total RPV
(mean ±SD, tmax: median [range])Postpartum
(6–12 Weeks)
(n=11)2nd Trimester of pregnancy
(n=15)3rd Trimester of pregnancy
(n=13)C0h, ng/mL 111 ± 69.2 65.0 ± 23.9 63.5 ± 26.2 Cmin, ng/mL 84.0 ± 58.8 54.3 ± 25.8 52.9 ± 24.4 Cmax, ng/mL 167 ± 101 121 ± 45.9 123 ± 47.5 tmax, h 4.00 (2.03−25.08) 4.00 (1.00−9.00) 4.00 (2.00−24.93) AUC24h, ng∙h/mL 2,714 ± 1,535 1,792 ± 711 1,762 ± 662 Drug Interaction Studies
Rilpivirine: RPV is primarily metabolized by cytochrome CYP3A, and drugs that induce or inhibit CYP3A may thus affect the clearance of RPV. Coadministration of COMPLERA and drugs that induce CYP3A may result in decreased plasma concentrations of RPV and loss of virologic response and possible resistance. Coadministration of COMPLERA and drugs that inhibit CYP3A may result in increased plasma concentrations of RPV. Coadministration of COMPLERA with drugs that increase gastric pH may result in decreased plasma concentrations of RPV and loss of virologic response and possible resistance to RPV and to the class of NNRTIs.
RPV at a dose of 25 mg once daily is not likely to have a clinically relevant effect on the exposure of medicinal products metabolized by CYP enzymes.
Emtricitabine and Tenofovir DF: In vitro and clinical pharmacokinetic drug-drug interaction studies have shown that the potential for CYP-mediated interactions involving FTC and tenofovir with other medicinal products is low.
FTC and tenofovir are primarily excreted by the kidneys by a combination of glomerular filtration and active tubular secretion. No drug-drug interactions due to competition for renal excretion have been observed; however, coadministration of FTC and TDF with drugs that are eliminated by active tubular secretion may increase concentrations of FTC, tenofovir, and/or the coadministered drug [see Drug Interactions (7.4, 7.6)].
Drugs that decrease renal function may increase concentrations of FTC and/or tenofovir.
The drug interaction studies described in Tables 9–14 were conducted with COMPLERA (RPV/FTC/TDF) or the components of COMPLERA (RPV, FTC, or TDF) administered individually.
The effects of coadministration of other drugs on the AUC, Cmax, and Cmin values of RPV, FTC, and TDF are summarized in Tables 9, 10, and 11, respectively. The effect of coadministration of RPV, FTC, and TDF on the AUC, Cmax, and Cmin values of other drugs are summarized in Tables 12, 13, and 14, respectively. For information regarding clinical recommendations, see Drug Interactions (7).
Table 9 Drug Interactions: Changes in Pharmacokinetic Parameters for RPV in the Presence of the Coadministered Drugs Coadministered Drug Dose of Coadministered Drug (mg) RPV Dose (mg) N* Mean % Change of RPV Pharmacokinetic Parameters†
(90% CI)Cmax AUC Cmin NA=not available - * N=maximum number of subjects for Cmax, AUC, or Cmin
- † Increase = ↑; Decrease = ↓; No Effect = ↔
- ‡ The interaction study has been performed with a dose higher than the recommended dose for RPV (25 mg once daily) assessing the maximal effect on the coadministered drug.
- § Study conducted with COMPLERA (RPV/FTC/TDF) coadministered with HARVONI (ledipasvir/sofosbuvir).
- ¶ Comparison based on historic controls.
- # Reference arm for comparison was 25 mg q.d. RPV administered alone.
- Þ Study conducted with COMPLERA coadministered with EPCLUSA (sofosbuvir/velpatasvir).
- ß Study conducted with ODEFSEY® (FTC/RPV/tenofovir alafenamide).
- à Study conducted with additional voxilaprevir 100 mg to achieve voxilaprevir exposures expected in HCV-infected patients.
Acetaminophen 500 single dose 150 once daily‡ 16 ↑9
(↑1 to ↑18)↑16
(↑10 to ↑22)↑26
(↑16 to ↑38)Atorvastatin 40 once daily 150 once daily‡ 16 ↓9
(↓21 to ↑6)↓10
(↓19 to ↓1)↓10
(↓16 to ↓4)Chlorzoxazone 500 single dose taken 2 hours after RPV 150 once daily‡ 16 ↑17
(↑8 to ↑27)↑25
(↑16 to ↑35)↑18
(↑9 to ↑28)Ethinyl Estradiol/
Norethindrone0.035 once daily/1 once daily 25 once daily 16 ↔§ ↔§ ↔§ Famotidine 40 single dose taken 12 hours before RPV 150 single dose‡ 24 ↓1
(↓16 to ↑16)↓9
(↓22 to ↑7)NA 40 single dose taken 2 hours before RPV 150 single dose‡ 23 ↓85
(↓88 to ↓81)↓76
(↓80 to ↓72)NA 40 single dose taken 4 hours after RPV 150 single dose‡ 24 ↑21
(↑6 to ↑39)↑13
(↑1 to ↑27)NA Ketoconazole 400 once daily 150 once daily‡ 15 ↑30
(↑13 to ↑48)↑49
(↑31 to ↑70)↑76
(↑57 to ↑97)Ledipasvir/
Sofosbuvir90/400 once daily 25 once daily§ 14 ↓3
(↓12 to ↑7)↑2
(↓6 to ↑11)↑12
(↑3 to ↑21)Methadone 60–100 once daily individualized dose 25 once daily 12 ↔¶ ↔¶ ↔¶ Omeprazole 20 once daily 150 once daily‡ 16 ↓40
(↓52 to ↓27)↓40
(↓49 to ↓29)↓33
(↓42 to ↓22)Rifabutin 300 once daily 25 once daily 18 ↓31
(↓38 to ↓24)↓42
(↓48 to ↓35)↓48
(↓54 to ↓41)300 once daily 50 once daily 18 ↑43
(↑30 to ↑56)#↑16
(↑6 to ↑26)#↓7
(↓15 to↑1)#Rifampin 600 once daily 150 once daily‡ 16 ↓69
(↓73 to ↓64)↓80
(↓82 to ↓77)↓89
(↓90 to ↓87)Simeprevir 25 once daily 150 once daily 23 ↑ 4
(↓ 5 to ↑ 13)↑ 12
(↑ 5 to ↑ 19)↑ 25
(↑ 16 to ↑ 35)Sildenafil 50 single dose 75 once daily 16 ↓8
(↓15 to ↓1)↓2
(↓8 to ↑5)↑4
(↓2 to ↑9)Sofosbuvir/
Velpatasvir400/100 once daily 25 once dailyÞ 24 ↓7
(↓12 to ↓2)↓5
(↓10 to 0)↓4
(↓10 to ↑3)Sofosbuvir/
Velpatasvir/
Voxilaprevirß400/100/100 + 100 voxilaprevirà once daily 25 once daily 30 ↓21
(↓26 to ↓16)↓20
(↓24 to ↓15)↓18
(↓23 to ↓13)TDF 300 once daily 150 once daily‡ 16 ↓4
(↓19 to ↑13)↑1
(↓13 to ↑18)↓1
(↓17 to ↑16)Table 10 Drug Interactions: Changes in Pharmacokinetic Parameters for FTC in the Presence of the Coadministered Drugs Coadministered Drug Dose of Coadministered Drug (mg) FTC Dose (mg) N* Mean % Change of FTC Pharmacokinetic Parameters†
(90% CI)Cmax AUC Cmin NA=not available - * N=maximum number of subjects for Cmax, AUC, or Cmin
- † Increase = ↑; Decrease = ↓; No Effect = ↔
- ‡ Study conducted with COMPLERA coadministered with HARVONI.
- § Study conducted with COMPLERA coadministered with EPCLUSA.
- ¶ Study conducted with additional voxilaprevir 100 mg to achieve voxilaprevir exposures expected in HCV-infected patients.
- # Study conducted with ODEFSEY (FTC/RPV/tenofovir alafenamide).
Famciclovir 500 × 1 200 × 1 12 ↔ ↔ NA Ledipasvir/
Sofosbuvir90/400 once daily 200 once daily‡ 15 ↑2
(↓2 to ↑6)↑5
(↑2 to ↑8)↑6
(↓3 to ↑15)Sofosbuvir/
Velpatasvir400/100 once daily 200 once daily§ 24 ↓5
(↓10 to 0)↓1
(↓3 to ↑2)↑5
(↓1 to ↑11)Sofosbuvir/
Velpatasvir/
Voxilaprevir400/100/100 + Voxilaprevir¶ 100 once daily 200 once daily# 30 ↓12
(↓17 to ↓7)↓7
(↓10 to ↓4)↑7
(↑1 to ↑14)TDF 300 once daily × 7 days 200 once daily × 7 days 17 ↔ ↔ ↑ 20
(↑ 12 to ↑ 29)Table 11 Drug Interactions: Changes in Pharmacokinetic Parameters for Tenofovir in the Presence of the Coadministered Drugs Coadministered Drug Dose of Coadministered Drug (mg) TDF Dose (mg)* N† Mean % Change of Tenofovir Pharmacokinetic Parameters‡
(90% CI)Cmax AUC Cmin NA=not available - * Subjects received VIREAD 300 mg daily.
- † N=maximum number of subjects for Cmax, AUC, or Cmin
- ‡ Increase = ↑; Decrease = ↓; No Effect = ↔
- § Study conducted with COMPLERA coadministered with HARVONI.
- ¶ Study conducted with COMPLERA coadministered with EPCLUSA.
Entecavir 1 once daily × 10 days 300 once daily ↔ ↔ ↔ Emtricitabine 200 once daily × 7 days 300 once daily × 7 days 17 ↔ ↔ ↔ Ledipasvir/
Sofosbuvir90/400 once daily × 10 days 300 once daily§ 14 ↑ 32
(↑ 25 to ↑ 39 )↑ 40
(↑ 31 to ↑ 50 )↑ 91
(↑ 74 to ↑ 110)Sofosbuvir/
Velpatasvir400/100 once daily 300 once daily 24 ↑ 44
(↑ 33 to ↑ 55)↑ 40
(↑ 34 to ↑ 46)↑ 84
(↑ 76 to ↑ 92)Tacrolimus 0.05 mg/kg twice daily × 7 days 300 once daily¶ 21 ↑ 13
(↑ 1 to ↑ 27)↔ ↔ Table 12 Drug Interactions: Changes in Pharmacokinetic Parameters for Coadministered Drugs in the Presence of RPV Coadministered Drug Dose of Coadministered Drug (mg) RPV Dose (mg) N* Mean % Change of Coadministered Drug Pharmacokinetic Parameters†
(90% CI)Cmax AUC Cmin NA = not available - * N=maximum number of subjects for Cmax, AUC, or Cmin
- † Increase = ↑; Decrease = ↓; No Effect = ↔
- ‡ The Interaction study has been performed with a dose higher than the recommended dose for RPV (25 mg once daily).
- § The predominant circulating nucleoside metabolite of sofosbuvir.
- ¶ Study conducted with ODEFSEY.
Acetaminophen 500 single dose 150 once daily‡ 16 ↓ 3
(↓ 14 to ↑ 10)↓ 8
(↓ 15 to ↓ 1)NA Atorvastatin 40 once daily 150 once daily‡ 16 ↑ 35
(↑ 8 to ↑ 68)↑ 4
(↓ 3 to ↑ 12)↓ 15
(↓ 31 to ↑ 3)2-hydroxy-atorvastatin 16 ↑ 58
(↑ 33 to ↑ 87)↑ 39
(↑ 29 to ↑ 50)↑ 32
(↑ 10 to ↑ 58)4-hydroxy-atorvastatin 16 ↑ 28
(↑ 15 to ↑ 43)↑ 23
(↑ 13 to ↑ 33)NA Chlorzoxazone 500 single dose taken 2 hours after RPV 150 once daily‡ 16 ↓ 2
(↓ 15 to ↑ 13)↑ 3
(↓ 5 to ↑ 13)NA Digoxin 0.5 single dose 25 once daily 22 ↑ 6
(↓ 3 to ↑ 17)↓ 2
(↓ 7 to ↑ 4)NA Ethinyl estradiol 0.035 once daily 25 once daily 17 ↑ 17
(↑ 6 to ↑ 30)↑ 14
(↑ 10 to ↑ 19)↑ 9
(↑ 3 to ↑ 16)Norethindrone 1 mg once daily ↓ 6
(↓ 17 to ↑ 6)↓ 11
(↓ 16 to ↓ 6)↓ 1
(↓ 10 to ↑ 8)Ketoconazole 400 once daily 150 once daily‡ 14 ↓ 15
(↓ 20 to ↓ 10)↓ 24
(↓ 30 to ↓ 18)↓ 66
(↓ 75 to ↓ 54)Ledipasvir 90 once daily 25 once daily 41 ↑ 1
(↓ 3 to ↑ 5)↑ 2
(↓ 3 to ↑ 6)↑ 2
(↓ 2 to ↑ 7)R(-) methadone 60−100 once daily individualized dose 25 once daily 13 ↓ 14
(↓ 22 to ↓ 5)↓ 16
(↓ 26 to ↓ 5)↓ 22
(↓ 33 to ↓ 9)S(+) methadone 13 ↓ 13
(↓ 22 to ↓ 3)↓ 16
(↓ 26 to ↓ 4)↓ 21
(↓ 33 to ↓ 8)Metformin 850 single dose 25 once daily 20 ↑ 2
(↓ 5 to ↑ 10)↓ 3
(↓ 10 to ↑ 6)NA Omeprazole 20 once daily 150 once daily‡ 15 ↓ 14
(↓ 32 to ↑ 9)↓ 14
(↓ 24 to ↓ 3)NA Rifampin 600 once daily 150 once daily‡ 16 ↑ 2
(↓ 7 to ↑ 12)↓ 1
(↓ 8 to ↑ 7)NA 25-desacetylrifampin 16 ↔
(↓ 13 to ↑ 15)↓ 9
(↓ 23 to ↑ 7)NA Simeprevir 150 once daily 25 once daily 21 ↑ 10
(↓ 3 to ↑ 26)↑ 6
(↓ 6 to ↑ 19)↓ 4
(↓ 17 to ↑ 11)Sildenafil 50 single dose 75 once daily‡ 16 ↓ 7
(↓ 20 to ↑ 8)↓ 3
(↓ 13 to ↑ 8)NA N-desmethyl-sildenafil ↓ 10
(↓ 20 to ↑ 2)↓ 8
(↓ 15 to ↓ 1)NA Sofosbuvir 400 once daily 25 once daily 24 ↑ 9
(↓ 5 to ↑ 25)↑ 16
(↑ 10 to ↑ 24)NA GS-331007§ ↓ 4
(↓ 10 to ↑ 1)↑ 4
(0 to ↑ 7)↑ 12
(↑ 7 to ↑ 17)Velpatasvir 100 once daily 25 once daily 24 ↓ 4
(↓ 15 to ↑ 10)↓ 1
(↓ 12 to ↑ 11)↑ 2
(↓ 9 to ↑ 15)Sofosbuvir 400 once daily 25 once daily¶ 30 ↓ 5
(↓ 14 to ↑ 5)↑ 1
(↓ 3 to ↑ 6)NA GS-331007§ ↑ 2
(↓ 2 to ↑ 6)↑ 4
(↑ 1 to ↑ 6)NA Velpatasvir 100 once daily 25 once daily¶ 30 ↑ 5
(↓ 4 to ↑ 16)↑ 1
(↓ 6 to ↑ 7)↑ 1
(↓ 5 to ↑ 9)Voxilaprevir 100 + 100 once daily 25 once daily¶ 30 ↓ 4
(↓ 16 to ↑ 11)↓ 6
(↓ 16 to ↑ 5)↑ 2
(↓ 8 to ↑ 12)TDF 300 once daily 150 once daily‡ 16 ↑ 19
(↑ 6 to ↑ 34)↑ 23
(↑ 16 to ↑ 31)↑ 24
(↑ 10 to ↑ 38)Table 13 Drug Interactions: Changes in Pharmacokinetic Parameters for Coadministered Drugs in the Presence of FTC Coadministered Drug Dose of Coadministered Drug (mg) FTC Dose (mg) N* Mean % Change of Coadministered Drug Pharmacokinetic Parameters†
(90% CI)Cmax AUC Cmin NA=not available - * All interaction trials conducted in healthy volunteers
- † No Effect = ↔
Famciclovir 500 × 1 200 × 1 12 ↔ ↔ NA TDF 300 once daily × 7 days 200 once daily × 7 days 17 ↔ ↔ ↔ No clinically significant drug interactions have been observed between FTC and indinavir, sofosbuvir/velpatasvir, sofosbuvir/velpatasvir/voxilaprevir, stavudine, and zidovudine.
Table 14 Drug Interactions: Changes in Pharmacokinetic Parameters for Coadministered Drugs in the Presence of TDF Coadministered Drug Dose of Coadministered Drug (mg) TDF Dose (mg) N* Mean % Change of Coadministered Drug Pharmacokinetic Parameters†
(90% CI)Cmax AUC Cmin NA=not available - * All interaction trials conducted in healthy volunteers
- † Increase = ↑; No Effect = ↔
Emtricitabine 200 once daily × 7 days 300 once daily × 7 days 17 ↔ ↔ ↑ 20
(↑ 12 to ↑ 29)Entecavir 1 once daily × 10 days 300 once daily 28 ↔ ↑ 13
(↑ 11 to ↑ 15)↔ Tacrolimus 0.05 mg/kg twice daily × 7 days 300 once daily 21 ↔ ↔ ↔ No effect on the pharmacokinetic parameters of the following coadministered drugs was observed with TDF: methadone, oral contraceptives (ethinyl estradiol/norgestimate), or ribavirin.
12.4 Microbiology
Mechanism of Action
Emtricitabine: FTC, a synthetic nucleoside analog of cytidine, is phosphorylated by cellular enzymes to form emtricitabine 5'-triphosphate. Emtricitabine 5'-triphosphate inhibits the activity of the HIV-1 RT by competing with the natural substrate deoxycytidine 5'-triphosphate and by being incorporated into nascent viral DNA, which results in chain termination. Emtricitabine 5′-triphosphate is a weak inhibitor of mammalian DNA polymerases α, β, ε, and mitochondrial DNA polymerase γ.
Rilpivirine: RPV is a diarylpyrimidine non-nucleoside reverse transcriptase inhibitor of HIV-1 and inhibits HIV-1 replication by non-competitive inhibition of HIV-1 RT. RPV does not inhibit the human cellular DNA polymerases α, β, and mitochondrial DNA polymerase γ.
Tenofovir DF: TDF is an acyclic nucleoside phosphonate diester analog of adenosine monophosphate. TDF requires initial diester hydrolysis for conversion to tenofovir and subsequent phosphorylations by cellular enzymes to form tenofovir diphosphate. Tenofovir diphosphate inhibits the activity of HIV-1 RT by competing with the natural substrate deoxyadenosine 5′-triphosphate and, after incorporation into DNA, by DNA chain termination. Tenofovir diphosphate is a weak inhibitor of mammalian DNA polymerases α, β, and mitochondrial DNA polymerase γ.
Antiviral Activity
Emtricitabine, Rilpivirine, and TDF: The triple combination of FTC, RPV, and TDF was not antagonistic in cell culture.
Emtricitabine: The antiviral activity of FTC against laboratory and clinical isolates of HIV-1 was assessed in lymphoblastoid cell lines, the MAGI-CCR5 cell line, and peripheral blood mononuclear cells. The 50% effective concentration (EC50) values for FTC were in the range of 0.0013–0.64 µM. FTC displayed antiviral activity in cell culture against HIV-1 clades A, B, C, D, E, F, and G (EC50 values ranged from 0.007–0.075 µM) and showed strain specific activity against HIV-2 (EC50 values ranged from 0.007–1.5 µM). In drug combination studies of FTC with nucleoside reverse transcriptase inhibitors (abacavir, lamivudine, stavudine, tenofovir, zidovudine), non-nucleoside reverse transcriptase inhibitors (delavirdine, EFV, nevirapine, and RPV), and protease inhibitors (amprenavir, nelfinavir, ritonavir, saquinavir), no antagonistic effects were observed.
Rilpivirine: RPV exhibited activity against laboratory strains of wild-type HIV-1 in an acutely infected T-cell line with a median EC50 value for HIV-1IIIB of 0.73 nM. RPV demonstrated limited activity in cell culture against HIV-2 with a median EC50 value of 5220 nM (range 2510–10,830 nM). RPV demonstrated antiviral activity against a broad panel of HIV-1 group M (subtype A, B, C, D, F, G, H) primary isolates with EC50 values ranging from 0.07–1.01 nM and was less active against group O primary isolates with EC50 values ranging from 2.88–8.45 nM. The antiviral activity of RPV was not antagonistic when combined with the NNRTIs EFV, etravirine, or nevirapine; the N(t)RTIs abacavir, didanosine, FTC, lamivudine, stavudine, tenofovir, or zidovudine; the PIs amprenavir, atazanavir, darunavir, indinavir, lopinavir, nelfinavir, ritonavir, saquinavir, or tipranavir; the gp41 fusion inhibitor enfuvirtide; the CCR5 co-receptor antagonist maraviroc; or the integrase strand transfer inhibitor raltegravir.
Tenofovir DF: The antiviral activity of tenofovir against laboratory and clinical isolates of HIV-1 was assessed in lymphoblastoid cell lines, primary monocyte/macrophage cells, and peripheral blood lymphocytes. The EC50 values for tenofovir were in the range of 0.04–8.5 µM. Tenofovir displayed antiviral activity in cell culture against HIV-1 clades A, B, C, D, E, F, G, and O (EC50 values ranged from 0.5–2.2 µM) and showed strain specific activity against HIV-2 (EC50 values ranged from 1.6–5.5 µM). In drug combination studies of tenofovir with NRTIs (abacavir, didanosine, FTC, lamivudine, stavudine, and zidovudine), NNRTIs (delavirdine, EFV, nevirapine, and RPV), and PIs (amprenavir, indinavir, nelfinavir, ritonavir, saquinavir), no antagonistic effects were observed.
Resistance
In Cell Culture
Emtricitabine and Tenofovir DF: HIV-1 isolates with reduced susceptibility to FTC or tenofovir have been selected in cell culture. Reduced susceptibility to FTC was associated with M184V/I substitutions in HIV-1 RT. HIV-1 isolates selected by tenofovir expressed a K65R substitution in HIV-1 RT and showed a 2–4 fold reduction in susceptibility to tenofovir. In addition, a K70E substitution in HIV-1 RT has been selected by tenofovir and results in low-level reduced susceptibility to abacavir, FTC, lamivudine, and tenofovir.
Rilpivirine: RPV-resistant strains were selected in cell culture starting from wild-type HIV-1 of different origins and subtypes as well as NNRTI-resistant HIV-1. The frequently observed amino acid substitutions that emerged and conferred decreased phenotypic susceptibility to RPV included: L100I, K101E, V106I and A, V108I, E138K and G, Q, R, V179F and I, Y181C and I, V189I, G190E, H221Y, F227C, and M230I and L.
In HIV-1-Infected Adult Subjects With No Antiretroviral Treatment History
In the Week 96 pooled resistance analysis for adult subjects receiving RPV or EFV in combination with FTC/TDF in the Phase 3 clinical trials C209 and C215, the emergence of resistance was greater among subjects' viruses in the RPV + FTC/TDF arm compared to the EFV + FTC/TDF arm and was dependent on baseline viral load. In the pooled resistance analysis, 61% (47/77) of the subjects who qualified for resistance analysis (resistance analysis subjects) in the RPV + FTC/TDF arm had virus with genotypic and/or phenotypic resistance to RPV compared to 42% (18/43) of the resistance analysis subjects in the EFV + FTC/TDF arm who had genotypic and/or phenotypic resistance to EFV. Moreover, genotypic and/or phenotypic resistance to FTC or tenofovir emerged in viruses from 57% (44/77) of the resistance analysis subjects in the RPV arm compared to 26% (11/43) in the EFV arm.
Emerging NNRTI substitutions in the RPV resistance analysis of subjects' viruses included V90I, K101E/P/T, E138K/A/Q/G, V179I/L, Y181C/I, V189I, H221Y, F227C/L, and M230L, which were associated with an RPV phenotypic fold change range of 2.6–621. The E138K substitution emerged most frequently during RPV treatment, commonly in combination with the M184I substitution. The FTC and lamivudine resistance-associated substitutions M184I or V and NRTI resistance-associated substitutions (K65R/N, A62V, D67N/G, K70E, Y115F, K219E/R) emerged more frequently in the RPV resistance analysis subjects than in EFV resistance analysis subjects (See Table 15).
NNRTI- and NRTI-resistance substitutions emerged less frequently in the resistance analysis of viruses from subjects with baseline viral loads of ≤100,000 copies/mL compared to viruses from subjects with baseline viral loads of >100,000 copies/mL: 23% (10/44) compared to 77% (34/44) of NNRTI-resistance substitutions and 20% (9/44) compared to 80% (35/44) of NRTI-resistance substitutions. This difference was also observed for the individual FTC/lamivudine and tenofovir resistance substitutions: 22% (9/41) compared to 78% (32/41) for M184I/V and 0% (0/8) compared to 100% (8/8) for K65R/N. Additionally, NNRTI and/or NRTI-resistance substitutions emerged less frequently in the resistance analysis of the viruses from subjects with baseline CD4+ cell counts ≥200 cells/mm3 compared to the viruses from subjects with baseline CD4+ cell counts <200 cells/mm3: 32% (14/44) compared to 68% (30/44) of NNRTI-resistance substitutions and 27% (12/44) compared to 73% (32/44) of NRTI-resistance substitutions.
Table 15 Proportion of Frequently Emerging Reverse Transcriptase Substitutions in the HIV-1 Virus of Resistance Analysis Adult Subjects* Who Received RPV or EFV in Combination with FTC/TDF from Pooled Phase 3 TMC278-C209 and TMC278-C215 Trials in the Week 96 Analysis C209 and C215
N=1096RPV+ FTC/TDF EFV+ FTC/TDF N=550 N=546 - * Subjects who qualified for resistance analysis
- † V90, L100, K101, K103, V106, V108, E138, V179, Y181, Y188, V189, G190, H221, P225, F227, and M230
- ‡ This combination of NRTI and NNRTI substitutions is a subset of those with the E138K.
- § A62V, K65R/N, D67N/G, K70E, L74I, Y115F, M184V/I, L210F, K219E/R
- ¶ These substitutions emerged in addition to the primary substitutions M184V/I or K65R; A62V (n=2), D67N/G (n=3), K70E (n=4), Y115F (n=2), K219E/R (n=8) in RPV resistance analysis subjects.
Subjects who Qualified for Resistance Analysis 14% (77/550) 8% (43/546) Subjects with Evaluable Postbaseline Resistance Data 70 31 Emergent NNRTI Substitutions† Any 63% (44/70) 55% (17/31) V90I 14% (10/70) 0 K101E/P/T/Q 19% (13/70) 10% (3/31) K103N 1% (1/70) 39% (12/31) E138K/A/Q/G 40% (28/70) 0 E138K+M184I‡ 30% (21/70) 0 V179I/D 6% (4/70) 10% (3/31) Y181C/I/S 13% (9/70) 3% (1/31) V189I 9% (6/70) 0 H221Y 10% (7/70) 0 Emergent NRTI Substitutions§ Any 63% (44/70) 32% (10/31) M184I/V 59% (41/70) 26% (8/31) K65R/N 11% (8/70) 6% (2/31) A62V, D67N/G, K70E, Y115F, or K219E/R¶ 20% (14/70) 3% (1/31) In Virologically Suppressed HIV-1-Infected Adult Subjects
Study 106: Through Week 48, 4 subjects who switched to COMPLERA (4 of 469 subjects, 0.9%) and 1 subject who maintained their ritonavir-boosted protease inhibitor-based regimen (1 of 159 subjects, 0.6%) developed genotypic and/or phenotypic resistance to a study drug. All 4 of the subjects who had resistance emergence on COMPLERA had evidence of FTC resistance and 3 of the subjects had evidence of RPV resistance.
Cross Resistance
Rilpivirine:
Site-Directed NNRTI Mutant Virus
Cross-resistance has been observed among NNRTIs. The single NNRTI substitutions K101P, Y181I, and Y181V conferred 52-fold, 15-fold, and 12-fold decreased susceptibility to RPV, respectively. The combination of E138K and M184I showed 6.7-fold reduced susceptibility to RPV compared to 2.8-fold for E138K alone. The K103N substitution did not show reduced susceptibility to RPV by itself. However, the combination of K103N and L100I resulted in a 7-fold reduced susceptibility to RPV. In another study, the Y188L substitution resulted in a reduced susceptibility to RPV of 9-fold for clinical isolates and 6-fold for site-directed mutants. Combinations of 2 or 3 NNRTI resistance-associated substitutions gave decreased susceptibility to RPV (fold change range of 3.7–554) in 38% and 66% of mutants, respectively.
In HIV-1-Infected Adult Subjects With No Antiretroviral Treatment History
Considering all available cell culture and clinical data, any of the following amino acid substitutions, when present at baseline, are likely to decrease the antiviral activity of RPV: K101E, K101P, E138A, E138G, E138K, E138R, E138Q, V179L, Y181C, Y181I, Y181V, Y188L, H221Y, F227C, M230I, M230L, and the combination of L100I+K103N.
Cross-resistance to EFV, etravirine, and/or nevirapine is likely after virologic failure and development of RPV resistance. In a pooled 96-week analysis for adult subjects receiving RPV in combination with FTC/TDF in the Phase 3 clinical trials TMC278-C209 and TMC278-C215, 43 of the 70 (61%) RPV resistance analysis subjects with postbaseline resistance data had virus with decreased susceptibility to RPV (≥2.5 fold change). Of these, 84% (n=36/43) were resistant to EFV (≥3.3-fold change), 88% (n=38/43) were resistant to etravirine (≥3.2-fold change), and 60% (n=26/43) were resistant to nevirapine (≥6-fold change). In the EFV arm, 3 of the 15 (20%) EFV resistance analysis subjects had viruses with resistance to etravirine and RPV, and 93% (14/15) had resistance to nevirapine. Virus from subjects experiencing virologic failure on RPV in combination with FTC/TDF developed more NNRTI resistance-associated substitutions conferring more cross-resistance to the NNRTI class and had a higher likelihood of cross-resistance to all NNRTIs in the class than subjects who failed on EFV.
Emtricitabine: FTC-resistant isolates (M184V/I) were cross-resistant to lamivudine but retained susceptibility in cell culture to didanosine, stavudine, tenofovir, zidovudine, and NNRTIs (delavirdine, EFV, nevirapine, and RPV). HIV-1 isolates containing the K65R substitution, selected in vivo by abacavir, didanosine, and tenofovir, demonstrated reduced susceptibility to inhibition by FTC. Viruses harboring substitutions conferring reduced susceptibility to stavudine and zidovudine (M41L, D67N, K70R, L210W, T215Y/F, K219Q/E), or didanosine (L74V), remained sensitive to FTC. HIV-1 containing the substitutions associated with NNRTI resistance K103N or RPV-associated substitutions were susceptible to FTC.
Tenofovir DF: The K65R and K70E substitutions selected by tenofovir are also selected in some HIV-1-infected patients treated with abacavir or didanosine. HIV-1 isolates with the K65R and K70E substitutions also showed reduced susceptibility to FTC and lamivudine. Therefore, cross-resistance among these NRTIs may occur in patients whose virus harbors the K65R substitution. HIV-1 isolates from patients (N=20) whose HIV-1 expressed a mean of 3 zidovudine-associated RT amino acid substitutions (M41L, D67N, K70R, L210W, T215Y/F, or K219Q/E/N) showed a 3.1-fold decrease in the susceptibility to tenofovir.
Subjects whose virus expressed an L74V substitution without zidovudine resistance-associated substitutions (N=8) had reduced response to TDF. Limited data are available for patients whose virus expressed a Y115F substitution (N=3), Q151M substitution (N=2), or T69 insertion (N=4), all of whom had a reduced response. HIV-1 containing the substitutions associated with NNRTI resistance K103N and Y181C, or RPV-associated substitutions, were susceptible to tenofovir.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Emtricitabine: In long-term carcinogenicity studies of FTC, no drug-related increases in tumor incidence were found in mice at doses up to 750 mg per kg per day (26 times the human systemic exposure at the therapeutic dose of 200 mg per day) or in rats at doses up to 600 mg per kg per day (31 times the human systemic exposure at the therapeutic dose).
FTC was not genotoxic in the reverse mutation bacterial test (Ames test), or the mouse lymphoma or mouse micronucleus assays.
FTC did not affect fertility in male rats at approximately 140-fold or in male and female mice at approximately 60-fold higher exposures (AUC) than in humans given the recommended 200 mg daily dose. Fertility was normal in the offspring of mice exposed daily from before birth (in utero) through sexual maturity at daily exposures (AUC) of approximately 60-fold higher than human exposures at the recommended 200 mg daily dose.
Rilpivirine: RPV was evaluated for carcinogenic potential by oral gavage administration to mice and rats up to 104 weeks. Daily doses of 20, 60, and 160 mg per kg per day were administered to mice and doses of 40, 200, 500, and 1500 mg per kg per day were administered to rats. In rats, there were no drug-related neoplasms. In mice, RPV was positive for hepatocellular neoplasms in both males and females. The observed hepatocellular findings in mice may be rodent-specific. At the lowest tested doses in the carcinogenicity studies, the systemic exposures (based on AUC) to RPV were 21 fold (mice) and 3 fold (rats), relative to those observed in humans at the recommended dose (25 mg once daily).
RPV has tested negative in the absence and presence of a metabolic activation system, in the in vitro Ames reverse mutation assay and in vitro clastogenicity mouse lymphoma assay. RPV did not induce chromosomal damage in the in vivo micronucleus test in mice.
In a study conducted in rats, there were no effects on mating or fertility with RPV up to 400 mg per kg per day, a dose of RPV that showed maternal toxicity. This dose is associated with an exposure that is approximately 40 times higher than the exposure in humans at the recommended dose of 25 mg once daily.
Tenofovir DF: Long-term oral carcinogenicity studies of TDF in mice and rats were carried out at exposures up to approximately 16 times (mice) and 5 times (rats) those observed in humans at the therapeutic dose for HIV-1 infection. At the high dose in female mice, liver adenomas were increased at exposures 16 times that in humans. In rats, the study was negative for carcinogenic findings at exposures up to 5 times that observed in humans at the therapeutic dose.
Tenofovir DF was mutagenic in the in vitro mouse lymphoma assay and negative in an in vitro bacterial mutagenicity test (Ames test). In an in vivo mouse micronucleus assay, TDF was negative when administered to male mice.
There were no effects on fertility, mating performance, or early embryonic development when TDF was administered to male rats at a dose equivalent to 10 times the human dose based on body surface area comparisons for 28 days prior to mating and to female rats for 15 days prior to mating through day 7 of gestation. There was, however, an alteration of the estrous cycle in female rats.
13.2 Animal Toxicology and/or Pharmacology
Tenofovir DF: Tenofovir and TDF administered in toxicology studies to rats, dogs, and monkeys at exposures (based on AUCs) greater than or equal to 6-fold those observed in humans caused bone toxicity. In monkeys the bone toxicity was diagnosed as osteomalacia. Osteomalacia observed in monkeys appeared to be reversible upon dose reduction or discontinuation of tenofovir. In rats and dogs, the bone toxicity manifested as reduced bone mineral density. The mechanism(s) underlying bone toxicity is unknown.
Evidence of renal toxicity was noted in 4 animal species. Increases in serum creatinine, BUN, glycosuria, proteinuria, phosphaturia, and/or calciuria and decreases in serum phosphate were observed to varying degrees in these animals. These toxicities were noted at exposures (based on AUCs) 2–20 times higher than those observed in humans. The relationship of the renal abnormalities, particularly the phosphaturia, to the bone toxicity is not known.
-
14 CLINICAL STUDIES
14.1 Adult Subjects
In HIV-1-Infected Adult Subjects With No Antiretroviral Treatment History
The efficacy of COMPLERA is based on the analyses of 48- and 96-week data from two randomized, double-blind, controlled studies (Study C209 [ECHO] and TRUVADA subset of Study C215 [THRIVE]) in treatment-naïve, HIV-1-infected subjects (N=1368). The studies are identical in design with the exception of the background regimen (BR). Subjects were randomized in a 1:1 ratio to receive either RPV 25 mg (N=686) once daily or EFV 600 mg (N=682) once daily in addition to a BR. In Study C209 (N=690), the BR was FTC/TDF. In Study C215 (N=678), the BR consisted of 2 NRTIs: FTC/TDF (60%, n=406), lamivudine/zidovudine (30%, n=204), or abacavir + lamivudine (10%, n=68).
For subjects who received FTC/TDF (N=1096) in studies C209 and C215, the mean age was 37 years (range 18–78), 78% were male, 62% were White, 24% were Black, and 11% were Asian. The mean baseline CD4+ cell count was 265 cells/mm3 (range 1–888) and 31% had CD4+ cell counts <200 cells/mm3. The median baseline plasma HIV-1 RNA was 5 log10 copies/mL (range 2–7). Subjects were stratified by baseline HIV-1 RNA. Fifty percent of subjects had baseline viral load ≤100,000 copies/mL, 39% of subjects had baseline viral load between 100,000 copies/mL to 500,000 copies/mL, and 11% of subjects had baseline viral load >500,000 copies/mL.
Treatment outcomes through 96 weeks for the subset of subjects receiving FTC/TDF in studies C209 and C215 (Table 16) are generally consistent with treatment outcomes for all participating subjects (presented in the prescribing information for Edurant). The incidence of virologic failure was higher in the RPV arm than the EFV arm at Week 96. Virologic failures and discontinuations due to adverse events mostly occurred in the first 48 weeks of treatment.
Table 16 Pooled Virologic Outcome of Randomized Treatment of Studies C209 and C215 at Week 96 in Adult Subjects With No Antiviral Treatment History in Combination with FTC/TDF) at Week 96* RPV+ FTC/TDF EFV+ FTC/TDF N=550 N=546 - * Analyses were based on the last observed viral load data within the Week 96 window (Week 90–103).
- † Predicted difference (95% CI) of response rate is 0.5% (–4.5% to 5.5%) at Week 96.
- ‡ Includes subjects who had ≥50 copies/mL in the Week 96 window, subjects who discontinued early due to lack or loss of efficacy, subjects who discontinued for reasons other than an adverse event, death, or lack or loss of efficacy and at the time of discontinuation had a viral load value of ≥50 copies/mL, and subjects who had a switch in background regimen that was not permitted by the protocol.
- § Includes subjects who discontinued due to an adverse event or death if this resulted in no on-treatment virologic data in the Week 96 window.
- ¶ Includes subjects who discontinued for reasons other than an adverse event, death, or lack or loss of efficacy, e.g., withdrew consent, loss to follow-up, etc.
HIV-1 RNA <50 copies/mL† 77% 77% HIV-1 RNA ≥50 copies/mL‡ 14% 8% No Virologic Data at Week 96 Window
ReasonsDiscontinued study due to adverse event or death§ 4% 9% Discontinued study for other reasons¶ and the last available HIV-1 RNA <50 copies/mL (or missing) 4% 6% Missing data during window but on study <1% <1% HIV-1 RNA <50 copies/mL
by Baseline HIV-1 RNA (copies/mL)≤100,000 83% 80% >100,000 71% 74% HIV-1 RNA ≥50 copies/mL‡
by Baseline HIV-1 RNA (copies/mL)≤100,000 7% 5% >100,000 22% 12% HIV-1 RNA <50 copies/mL
by Baseline CD4+ Cell Count (cells/mm3)<200 68% 72% ≥200 82% 79% HIV-1 RNA ≥50 copies/mL‡
by Baseline CD4+ Cell Count (cells/mm3)<200 27% 12% ≥200 8% 7% Based on the pooled data from studies C209 and C215, the mean CD4+ cell count increase from baseline at Week 96 was 226 cells/mm3 for RPV + FTC/TDF-treated subjects and 223 cells/mm3 for EFV + FTC/TDF-treated subjects.
In Virologically Suppressed HIV-1-Infected Adult Subjects
The efficacy and safety of switching from a ritonavir-boosted protease inhibitor in combination with two NRTIs to COMPLERA was evaluated in Study 106, a randomized, open-label study in virologically suppressed HIV-1-infected adults. Subjects had to be on either their first or second antiretroviral regimen with no history of virologic failure, have no current or past history of resistance to any of the three components of COMPLERA, and must have been suppressed (HIV-1 RNA <50 copies/mL) for at least 6 months prior to screening. Subjects were randomized in a 2:1 ratio to either switch to COMPLERA at baseline (COMPLERA arm, N=317), or stay on their baseline antiretroviral regimen for 24 weeks (SBR arm, N=159) and then switch to COMPLERA for an additional 24 weeks (N=52). Subjects had a mean age of 42 years (range 19–73), 88% were male, 77% were White, 17% were Black, and 17% were Hispanic/Latino. The mean baseline CD4+ cell count was 584 cells/mm3 (range 42–1484). Randomization was stratified by use of TDF and/or lopinavir/ritonavir in the baseline regimen.
Treatment outcomes are presented in Table 17.
Table 17 Virologic Outcomes of Study GS-US-264-0106 in Virologically Suppressed Subjects COMPLERA Stayed on Baseline Regimen (SBR) Week 48* Week 24† N=317 N=159 - * Week 48 window is between Day 295 and 378 (inclusive).
- † For subjects in the SBR arm who maintained their baseline regimen for 24 weeks and then switched to COMPLERA, the Week 24 window is between Day 127 and first dose day on COMPLERA.
- ‡ Predicted difference (95% CI) of response rate for switching to COMPLERA at Week 48 compared to staying on baseline regimen at Week 24 (in absence of Week 48 results from the SBR group by study design) is –0.7% (–6.4% to 5.1%).
- § Includes subjects who had HIV-1 RNA ≥50 copies/mL in the time window, subjects who discontinued early due to lack or loss of efficacy, and subjects who discontinued for reasons other than an adverse event or death and at the time of discontinuation had a viral load value of ≥50 copies/mL.
- ¶ Includes subjects who discontinued due to adverse event or death at any time point from Day 1 through the time window if this resulted in no virologic data on treatment during the specified window.
- # Includes subjects who discontinued for reasons other than an adverse event, death, or lack or loss of efficacy, e.g., withdrew consent, loss to follow-up, etc.
HIV-1 RNA <50 copies/mL‡ 89% (283/317) 90% (143/159) HIV-1 RNA ≥50 copies/mL§ 3% (8/317) 5% (8/159) No Virologic Data at Week 24 Window Discontinued study drug due to AE or death¶ 2% (7/317) 0% Discontinued study drug due to other reasons and last available HIV-1 RNA <50 copies/mL# 5% (16/317) 3% (5/159) Missing data during window but on study drug 1% (3/317) 2% (3/159) 14.2 Pediatric Subjects
The pharmacokinetics, safety, and efficacy of RPV in combination with other antiretroviral agents was evaluated in a single-arm, open-label Phase 2 trial in antiretroviral treatment-naïve HIV-1-infected pediatric subjects 12 to less than 18 years of age and weighing at least 32 kg (TMC-C213). Thirty-six (36) subjects were enrolled with a median age of 14.5 years (range 12 to 17 years), and were 55.6% female, 88.9% Black, and 11.1% Asian. The majority of subjects (24/36) received RPV in combination with FTC and TDF. Of these 24 subjects, 20 had baseline HIV RNA ≤100,000 copies/mL. The baseline characteristics and efficacy outcomes at Week 48 are further described below for the 20 subjects.
The median baseline plasma HIV-1 RNA and CD4+ cell count were 49,550 (range 2060 to 92,600 copies/mL) and 437.5 cells/mm3 (range 123 to 983 cells/mm3), respectively. At Week 48, 80% (16/20) of the subjects had HIV RNA <50 copies/mL, 15% (3/20) had HIV RNA ≥50 copies/mL, and one subject discontinued therapy prior to Week 48 and before reaching virologic suppression (HIV RNA <50 copies/mL). At Week 48, the mean increase in CD4+ cell count from baseline was 225 cells/mm3.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
COMPLERA tablets are purplish pink, capsule shaped, film coated, debossed with "GSI" on one side, and plain faced on the other side. Each bottle contains 30 tablets (NDC: 61958-1101-1), a silica gel desiccant, and a polyester fiber coil, and is closed with a child-resistant closure.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Posttreatment Acute Exacerbation of Hepatitis B in Patients Coinfected with HIV-1 and HBV
Severe acute exacerbations of hepatitis B have been reported in patients who are coinfected with HBV and HIV-1 and who have discontinued products containing FTC or TDF [see Warnings and Precautions (5.1)]. Advise patients to not discontinue COMPLERA without first informing their healthcare provider.
Severe Skin Reactions and Hypersensitivity
Advise patients to immediately contact their healthcare provider if they develop a rash. Instruct patients to immediately stop taking COMPLERA and seek medical attention if they develop a rash associated with any of the following symptoms, as it may be a sign of a more serious reaction such as DRESS severe hypersensitivity: fever, blisters, mucosal involvement, eye inflammation (conjunctivitis), severe allergic reaction causing swelling of the face, eyes, lips, mouth, tongue, or throat which may lead to difficulty swallowing or breathing, and any signs and symptoms of liver problems, as they may be a sign of a more serious reaction. Patients should understand that if severe rash occurs, they will be closely monitored, laboratory tests will be performed and appropriate therapy will be initiated [see Warnings and Precautions (5.2)].
Hepatotoxicity
Inform patients that hepatotoxicity has been reported with COMPLERA and that monitoring for hepatotoxicity is recommended [see Warnings and Precautions (5.3)].
Depressive Disorders
Inform patients that depressive disorders (depressed mood, depression, dysphoria, major depression, mood altered, negative thoughts, suicide attempt, suicidal ideation) have been reported with COMPLERA. Advise patients to seek immediate medical evaluation if they experience depressive symptoms [see Warnings and Precautions (5.4)].
New Onset or Worsening Renal Impairment
Inform patients that renal impairment, including cases of acute renal failure and Fanconi syndrome, has been reported in association with the use of TDF. COMPLERA should be avoided with concurrent or recent use of a nephrotoxic agent (e.g., high-dose or multiple NSAIDs) [see Warnings and Precautions (5.5)].
Bone Loss and Mineralization Defects
Inform patients that decreases in bone mineral density have been observed with the use of TDF. Assessment of bone mineral density (BMD) should be considered in patients who have a history of pathologic bone fracture or other risk factors for osteoporosis or bone loss [see Warnings and Precautions (5.6)].
Drug Interactions
COMPLERA may interact with many drugs and is not recommended to be coadministered with numerous drugs. Advise patients to report to their healthcare provider the use of any other prescription or nonprescription medication or herbal products, including St. John's wort [see Contraindications (4), Warnings and Precautions (5.7), and Drug Interactions (7)].
For patients receiving rifabutin, an additional 25 mg tablet of RPV (Edurant) once per day is recommended to be taken concomitantly with COMPLERA and with a meal for the duration of rifabutin coadministration.
Lactic Acidosis and Severe Hepatomegaly
Inform patients that lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported. Treatment with COMPLERA should be suspended in any patient who develops clinical symptoms suggestive of lactic acidosis or pronounced hepatotoxicity [see Warnings and Precautions (5.8)].
Immune Reconstitution Syndrome
Inform patients to inform their healthcare provider immediately of any signs and symptoms of inflammation from previous infections, which may occur soon after anti-HIV treatment is started [see Warnings and Precautions (5.9)].
Dosing Instructions
Advise patients that it is important to take COMPLERA on a regular dosing schedule with food and to avoid missing doses. A protein drink is not a substitute for food. If the healthcare provider decides to stop COMPLERA and the patient is switched to new medicines to treat HIV that include RPV tablets, the RPV tablets should be taken only with a meal.
Pregnancy Registry
Inform patients that there is an antiretroviral pregnancy registry to monitor fetal outcomes in those exposed to COMPLERA during pregnancy [see Use in Specific Populations (8.1)].
Lactation
Instruct patients with HIV-1 infection not to breastfeed because HIV-1 can be passed to the baby in breast milk [see Use in Specific Populations (8.2)].
-
SPL UNCLASSIFIED SECTION
Manufactured and distributed by:
Gilead Sciences, Inc.
Foster City, CA 94404COMPLERA, EMTRIVA, EPCLUSA, GSI, HARVONI, ODEFSEY, TRUVADA, VIREAD, and VOSEVI are trademarks of Gilead Sciences, Inc., or its related companies. All other trademarks referenced herein are the property of their respective owners.
© 2019 Gilead Sciences, Inc. All rights reserved.
202123-GS-013
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 11/2019 Patient Information
COMPLERA® (kom-PLEH-rah)
(emtricitabine, rilpivirine, tenofovir disoproxil fumarate)
tabletsImportant: Ask your healthcare provider or pharmacist about medicines that should not be taken with COMPLERA. For more information, see the section "What should I tell my healthcare provider before taking COMPLERA?" What is the most important information I should know about COMPLERA?
COMPLERA can cause serious side effects, including:
Worsening of Hepatitis B virus (HBV) infection. Your healthcare provider will test you for HBV before starting treatment with COMPLERA. If you have HBV infection and take COMPLERA, your HBV may get worse (flare-up) if you stop taking COMPLERA. A "flare-up" is when your HBV infection suddenly returns in a worse way than before.- Do not stop taking COMPLERA without first talking to your healthcare provider.
- Do not run out of COMPLERA. Refill your prescription or talk to your healthcare provider before your COMPLERA is all gone.
- If you stop taking COMPLERA, your healthcare provider will need to check your health often and do blood tests regularly for several months to check your HBV infection. Tell your healthcare provider about any new or unusual symptoms you may have after you stop taking COMPLERA.
What is COMPLERA?
COMPLERA is a prescription medicine that is used to treat Human Immunodeficiency Virus-1 (HIV-1) in people weighing at least 77 lb (35 kg) who:- have never taken HIV-1 medicines before, and who have an amount of HIV-1 in their blood (this is called 'viral load') that is no more than 100,000 copies/mL before they start taking COMPLERA,
or - in certain people who have a viral load that is less than 50 copies/mL when they start taking COMPLERA, to replace their current HIV-1 medicines.
COMPLERA contains 3 medicines (emtricitabine, rilpivirine, tenofovir disoproxil fumarate) combined in one tablet. Emtricitabine (EMTRIVA®) and tenofovir disoproxil fumarate (VIREAD®) are HIV-1 nucleoside analog reverse transcriptase inhibitors (NRTIs). Rilpivirine (Edurant®) is an HIV-1 non-nucleoside analog reverse transcriptase inhibitor (NNRTI).
It is not known if COMPLERA is safe and effective in children less than 12 years of age or who weigh less than 77 lb (35 kg).Who should not take COMPLERA?
Do not take COMPLERA if you also take:- anti-seizure medicines:
- carbamazepine
- oxcarbazepine
- phenobarbital
- phenytoin
- anti-tuberculosis (anti-TB) medicines:
- rifampin
- rifapentine
- proton pump inhibitor (PPI) medicine for certain stomach or intestinal problems:
- dexlansoprazole
- esomeprazole
- lansoprazole
- omeprazole
- pantoprazole sodium
- rabeprazole
- more than 1 dose of the steroid medicine dexamethasone or dexamethasone sodium phosphate
- St. John's wort (Hypericum perforatum)
What should I tell my healthcare provider before taking COMPLERA?
Before taking COMPLERA, tell your healthcare provider about all your medical conditions, including if you:- have liver problems, including hepatitis B or C virus infection
- have kidney problems
- have a history of depression or suicidal thoughts
- have bone problems
- are pregnant or plan to become pregnant. It is not known if COMPLERA can harm your unborn child. Tell your healthcare provider if you become pregnant during treatment with COMPLERA.
Pregnancy Registry. There is a pregnancy registry for those who take COMPLERA during pregnancy. The purpose of this registry is to collect information about the health of you and your baby. Talk with your healthcare provider about how you can take part in this registry. - are breastfeeding or plan to breastfeed. Do not breastfeed if you are taking COMPLERA.
- You should not breastfeed if you have HIV-1 because of the risk of passing HIV-1 to your baby.
- At least two of the medicines contained in COMPLERA can be passed to your baby in your breast milk.
- Talk with your healthcare provider about the best way to feed your baby during treatment with COMPLERA.
Some medicines interact with COMPLERA. Keep a list of your medicines to show your healthcare provider and pharmacist when you get a new medicine.- You can ask your healthcare provider or pharmacist for a list of medicines that can interact with COMPLERA.
- Do not start taking a new medicine without telling your healthcare provider. Your healthcare provider can tell you if it is safe to take COMPLERA with other medicines.
How should I take COMPLERA? - Take COMPLERA exactly as your healthcare provider tells you to take it.
- Take COMPLERA with food. Taking COMPLERA with food is important to help get the right amount of medicine in your body. A protein drink does not replace food. If your healthcare provider decides to stop COMPLERA and you are switched to new medicines to treat HIV-1 that include rilpivirine tablets, the rilpivirine tablets should be taken only with a meal.
- Do not change your dose or stop taking COMPLERA without first talking with your healthcare provider. Stay under the care of your healthcare provider during treatment with COMPLERA.
- If you miss a dose of COMPLERA within 12 hours of the time you usually take it, take your dose of COMPLERA with food as soon as possible. Then, take your next dose of COMPLERA at the regularly scheduled time. If you miss a dose of COMPLERA by more than 12 hours of the time you usually take it, wait and then take the next dose of COMPLERA at the regularly scheduled time.
- Do not take more than your prescribed dose to make up for a missed dose.
- If you take too much COMPLERA, contact your local poison control center or go to the nearest hospital emergency room right away.
- When your COMPLERA supply starts to run low, get more from your healthcare provider or pharmacy. It is very important not to run out of COMPLERA. The amount of virus in your blood may increase if the medicine is stopped for even a short time.
What are the possible side effects of COMPLERA?
COMPLERA can cause serious side effects, including:-
See "What is the most important information I should know about COMPLERA?"
Severe skin rash and allergic reactions. Skin rash is a common side effect of COMPLERA. Rash can be serious. Call your healthcare provider right away if you get a rash. In some cases, rash and allergic reaction may need to be treated in a hospital. If you get a rash with any of the following symptoms, stop taking COMPLERA and call your healthcare provider or get medical help right away:
- fever
- skin blisters
- mouth sores
- redness or swelling of the eyes (conjunctivitis)
- swelling of the face, lips, mouth, tongue or throat
- trouble breathing or swallowing
- pain on the right side of the stomach (abdominal) area
- dark or "tea colored" urine
- Severe liver problems. In rare cases, severe liver problems can happen that can lead to death. Tell your healthcare provider right away if you get these symptoms: skin or the white part of your eyes turns yellow, dark "tea-colored" urine, light-colored stools, loss of appetite for several days or longer, nausea, or stomach-area pain.
- Change in liver enzymes. People with a history of hepatitis B or C virus infection or who have certain liver enzyme changes may have an increased risk of developing new or worsening liver problems during treatment with COMPLERA. Liver problems can also happen during treatment with COMPLERA in people without a history of liver disease. Your healthcare provider may need to do tests to check your liver enzymes before and during treatment with COMPLERA.
-
Depression or mood changes. Tell your healthcare provider right away if you have any of the following symptoms:
- feel sad or hopeless
- feel anxious or restless
- have thoughts of hurting yourself (suicide) or have tried to hurt yourself
- New or worse kidney problems, including kidney failure, can happen in some people who take COMPLERA. Your healthcare provider should do blood tests to check your kidneys before starting treatment with COMPLERA. If you have had kidney problems in the past or need to take another medicine that can cause kidney problems, your healthcare provider may need to do blood tests to check your kidneys during your treatment with COMPLERA.
- Bone problems can happen in some people who take COMPLERA. Bone problems include bone pain, softening, or thinning (which may lead to fractures). Your healthcare provider may need to do additional tests to check your bones.
- Too much lactic acid in your blood (lactic acidosis). Too much lactic acid is a serious but rare medical emergency that can lead to death. Tell your healthcare provider right away if you get these symptoms: weakness or being more tired than usual, unusual muscle pain, being short of breath or fast breathing, stomach pain with nausea and vomiting, cold or blue hands and feet, feel dizzy or lightheaded, or a fast or abnormal heartbeat.
- Changes in your immune system (Immune Reconstitution Syndrome) can happen when you start taking HIV-1 medicines. Your immune system may get stronger and begin to fight infections that have been hidden in your body for a long time. Tell your healthcare provider right away if you start having new symptoms after starting your HIV-1 medicine.
- depression
- trouble sleeping
- headache
- diarrhea
- nausea
- tiredness
- headache
- dizziness
- depression
- trouble sleeping
- abnormal dreams
- rash
These are not all the possible side effects of COMPLERA.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store COMPLERA? - Store COMPLERA at room temperature between 68 °F to 77 °F (20 °C to 25 °C).
- Keep COMPLERA in its original container and keep the container tightly closed.
- Do not use COMPLERA if the seal over the bottle opening is broken or missing.
General information about safe and effective use of COMPLERA
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use COMPLERA for a condition for which it was not prescribed. Do not give COMPLERA to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about COMPLERA that is written for health professionals.
For more information, call 1-800-445-3235 or go to www.COMPLERA.com.What are the ingredients of COMPLERA?
Active ingredients: emtricitabine, rilpivirine hydrochloride, and tenofovir disoproxil fumarate.
Inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polysorbate 20 povidone, pregelatinized starch. The tablet film coating contains FD&C Blue #2 aluminum lake, FD&C Yellow #6 aluminum lake, hypromellose, iron oxide red, lactose monohydrate, polyethylene glycol, titanium dioxide, triacetin.
Manufactured and distributed by: Gilead Sciences, Inc. Foster City, CA 94404
COMPLERA, EMTRIVA, and VIREAD are trademarks of Gilead Sciences, Inc., or its related companies. All other trademarks referenced herein are the property of their respective owners.
© 2019 Gilead Sciences, Inc. All rights reserved.
202123-GS-013 -
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 30 Tablet Bottle Label
NDC: 61958-1101-1
30 tabletsCOMPLERA®
(emtricitabine, rilpivirine,
tenofovir disoproxil fumarate) Tablets
200 mg/25 mg/300 mgNote to pharmacist: Do not cover ALERT box with pharmacy label.
ALERT: Find out about medicines that
should NOT be taken with COMPLERA®
-
INGREDIENTS AND APPEARANCE
COMPLERA
emtricitabine, rilpivirine hydrochloride, and tenofovir disoproxil fumarate tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 61958-1101 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength emtricitabine (UNII: G70B4ETF4S) (emtricitabine - UNII:G70B4ETF4S) emtricitabine 200 mg rilpivirine hydrochloride (UNII: 212WAX8KDD) (rilpivirine - UNII:FI96A8X663) rilpivirine 25 mg tenofovir disoproxil fumarate (UNII: OTT9J7900I) (tenofovir anhydrous - UNII:W4HFE001U5) tenofovir disoproxil fumarate 300 mg Inactive Ingredients Ingredient Name Strength microcrystalline cellulose (UNII: OP1R32D61U) lactose monohydrate (UNII: EWQ57Q8I5X) Povidone, Unspecified (UNII: FZ989GH94E) polysorbate 20 (UNII: 7T1F30V5YH) croscarmellose sodium (UNII: M28OL1HH48) magnesium stearate (UNII: 70097M6I30) starch, corn (UNII: O8232NY3SJ) hypromellose, unspecified (UNII: 3NXW29V3WO) FD&C Blue No. 2 (UNII: L06K8R7DQK) aluminum oxide (UNII: LMI26O6933) polyethylene glycol, unspecified (UNII: 3WJQ0SDW1A) ferric oxide red (UNII: 1K09F3G675) titanium dioxide (UNII: 15FIX9V2JP) triacetin (UNII: XHX3C3X673) FD&C Yellow No. 6 (UNII: H77VEI93A8) water (UNII: 059QF0KO0R) Product Characteristics Color PURPLE (Purplish-Pink) Score no score Shape OVAL (capsule-shaped) Size 19mm Flavor Imprint Code GSI Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 61958-1101-1 30 in 1 BOTTLE; Type 0: Not a Combination Product 08/10/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA202123 08/10/2011 Labeler - Gilead Sciences, Inc. (185049848)
Trademark Results [COMPLERA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 COMPLERA 77763051 4038955 Live/Registered |
GILEAD SCIENCES IRELAND UC 2009-06-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.