EMTRIVA- emtricitabine capsule EMTRIVA- emtricitabine solution
Emtriva by
Drug Labeling and Warnings
Emtriva by is a Prescription medication manufactured, distributed, or labeled by Gilead Sciences, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use EMTRIVA safely and effectively. See full prescribing information for EMTRIVA.
EMTRIVA® (emtricitabine) capsule, for oral use
EMTRIVA® (emtricitabine) oral solution
Initial U.S. Approval: 2003WARNING: POSTTREATMENT ACUTE EXACERBATION OF HEPATITIS B
See full prescribing information for complete boxed warning.
Severe acute exacerbations of Hepatitis B (HBV) have been reported in patients coinfected with HIV-1 and HBV who have discontinued EMTRIVA. Hepatic function should be monitored closely in patients coinfected with HIV-1 and HBV who discontinue EMTRIVA. If appropriate, initiation of anti-hepatitis B therapy may be warranted. (5.1)
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
EMTRIVA, a nucleoside analog HIV-1 reverse transcriptase inhibitor, is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection. (1)
DOSAGE AND ADMINISTRATION
- Testing: Prior to or when initiating EMTRIVA test for hepatitis B virus infection. (2.1)
- EMTRIVA may be taken without regard to food. (2.2)
- Adult Patients (18 years of age and older) (2.3):
- EMTRIVA capsules: One 200 mg capsule administered once daily orally.
- EMTRIVA oral solution: 240 mg (24 mL) administered once daily orally.
- Pediatric Patients (0–3 months of age) (2.4):
- EMTRIVA oral solution: 3 mg/kg administered once daily orally.
- Pediatric Patients (3 months through 17 years of age) (2.5):
- EMTRIVA capsules: For children weighing more than 33 kg who can swallow an intact capsule, one 200 mg capsule administered once daily orally.
- EMTRIVA oral solution: 6 mg/kg up to a maximum of 240 mg (24 mL) administered once daily orally.
- Dose interval adjustment in adult patients with renal impairment (2.6):
Creatinine Clearance (mL/min) Formulation ≥50 mL/min 30–49 mL/min 15–29 mL/min <15 mL/min or on hemodialysis* - * Hemodialysis Patients: If dosing on day of dialysis, give dose after dialysis.
Capsule
(200 mg)200 mg every 24 hours 200 mg every 48 hours 200 mg every 72 hours 200 mg every 96 hours Oral Solution
(10 mg/mL)240 mg every 24 hours
(24 mL)120 mg every 24 hours
(12 mL)80 mg every 24 hours
(8 mL)60 mg every 24 hours
(6 mL)CONTRAINDICATIONS
EMTRIVA is contraindicated in patients with previously demonstrated hypersensitivity to any of the components of the products. (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥10%) are headache, diarrhea, nausea, fatigue, dizziness, depression, insomnia, abnormal dreams, rash, abdominal pain, asthenia, increased cough, and rhinitis. Skin hyperpigmentation was very common (≥10%) in pediatric patients. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Gilead Sciences, Inc. at 1-800-GILEAD-5 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: POSTTREATMENT ACUTE EXACERBATION OF HEPATITIS B
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Testing Prior to Initiation of Treatment with EMTRIVA
2.2 Recommended Dosage
2.3 Recommended Dosage in Adult Patients (18 years of age and older)
2.4 Recommended Dosage in Pediatric Patients (0–3 months of age)
2.5 Recommended Dosage in Pediatric Patients (3 months through 17 years of age)
2.6 Dosage Adjustment in Patients with Renal Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Severe Acute Exacerbation of Hepatitis B in Patients Coinfected with HIV-1 and HBV
5.2 Immune Reconstitution Syndrome
5.3 Lactic Acidosis/Severe Hepatomegaly with Steatosis
5.4 Dose Adjustment in Patients with New Onset or Worsening Renal Impairment
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Overview of Clinical Trials
14.2 Clinical Trial Results in Treatment-Naïve Adults
14.3 Clinical Trial Results in Treatment-Experienced Adults
14.4 Clinical Trial Results in Pediatrics
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: POSTTREATMENT ACUTE EXACERBATION OF HEPATITIS B
Severe acute exacerbations of hepatitis B (HBV) have been reported in patients who are coinfected with HIV-1 and HBV and have discontinued EMTRIVA. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who are coinfected with HIV-1 and HBV and discontinue EMTRIVA. If appropriate, initiation of anti-hepatitis B therapy may be warranted [see Warnings and Precautions (5.1)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Testing Prior to Initiation of Treatment with EMTRIVA
Prior to or when initiating EMTRIVA, test patients for hepatitis B virus infection [see Warnings and Precautions (5.1)].
2.2 Recommended Dosage
EMTRIVA is taken by mouth once daily and may be taken without regard to food [see Clinical Pharmacology (12.3)].
2.3 Recommended Dosage in Adult Patients (18 years of age and older)
EMTRIVA capsules: One 200 mg capsule administered once daily orally.
EMTRIVA oral solution: 240 mg (24 mL) administered once daily orally.
2.4 Recommended Dosage in Pediatric Patients (0–3 months of age)
EMTRIVA oral solution: 3 mg per kg administered once daily orally.
2.5 Recommended Dosage in Pediatric Patients (3 months through 17 years of age)
EMTRIVA oral solution: 6 mg per kg up to a maximum of 240 mg (24 mL) administered once daily orally.
EMTRIVA capsules: For pediatric patients weighing more than 33 kg who can swallow an intact capsule, one 200 mg capsule administered once daily orally.
2.6 Dosage Adjustment in Patients with Renal Impairment
Table 1 provides dosage interval adjustment for patients with renal impairment. No dosage adjustment is necessary for patients with mild renal impairment (creatinine clearance 50–80 mL/min). The safety and effectiveness of dose adjustment recommendations in patients with moderate to severe renal impairment (creatinine clearance below 50 mL/min) have not been clinically evaluated. Therefore, clinical response to treatment and renal function should be closely monitored in these patients [see Warnings and Precautions (5.4), Use in Specific Populations (8.6)].
Table 1 Dose Interval Adjustment for Adult Patients with Altered Creatinine Clearance Creatinine Clearance (mL/min) Formulation ≥50 mL/min 30–49 mL/min 15–29 mL/min <15 mL/min or on hemodialysis* - * Hemodialysis Patients: If dosing on day of dialysis, give dose after dialysis.
Capsule
(200 mg)200 mg every 24 hours 200 mg every 48 hours 200 mg every 72 hours 200 mg every 96 hours Oral Solution
(10 mg/mL)240 mg every 24 hours
(24 mL)120 mg every 24 hours
(12 mL)80 mg every 24 hours
(8 mL)60 mg every 24 hours
(6 mL)There are insufficient data available to make dosage recommendations in pediatric patients with renal impairment.
-
3 DOSAGE FORMS AND STRENGTHS
EMTRIVA is available as capsules or as an oral solution.
- 200 mg Capsules: 200 mg of emtricitabine (FTC): size 1 hard gelatin capsules with a blue cap and white body, printed with "200 mg" in black on the cap and with "GILEAD" and the corporate logo in black on the body.
- Oral solution: clear, orange to dark orange liquid containing 10 mg of FTC per mL.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Severe Acute Exacerbation of Hepatitis B in Patients Coinfected with HIV-1 and HBV
All patients should be tested for the presence of chronic Hepatitis B virus (HBV) before or when initiating EMTRIVA [see Dosage and Administration (2.1)].
Severe acute exacerbations of hepatitis B (e.g., liver decompensation and liver failure) have been reported in patients who are coinfected with HIV-1 and HBV and have discontinued EMTRIVA. Patients who are coinfected with HIV-1 and HBV who discontinue EMTRIVA should be closely monitored with both clinical and laboratory follow-up for at least several months after stopping treatment. If appropriate, initiation of anti-hepatitis B therapy may be warranted, especially in patients with advanced liver disease or cirrhosis, since posttreatment exacerbation of hepatitis may lead to hepatic decompensation and liver failure.
5.2 Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including EMTRIVA. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia [PCP], or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves' disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable, and can occur many months after initiation of treatment.
5.3 Lactic Acidosis/Severe Hepatomegaly with Steatosis
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs, including FTC, alone or in combination with other antiretrovirals. Treatment with EMTRIVA should be suspended in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
5.4 Dose Adjustment in Patients with New Onset or Worsening Renal Impairment
Emtricitabine is principally eliminated by the kidney. Reduction of the dosage of EMTRIVA is recommended for patients with impaired renal function [see Dosage and Administration (2.6), Use in Specific Populations (8.6), and Clinical Pharmacology (12.3)].
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in other sections of the labeling:
- Severe Acute Exacerbation of Hepatitis B in Patients Coinfected with HIV-1 and HBV [see Warnings and Precautions (5.1)].
- Immune Reconstitution Syndrome [see Warnings and Precautions (5.2)].
- Lactic Acidosis/Severe Hepatomegaly with Steatosis [see Warnings and Precautions (5.3)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions from Clinical Trials Experience in Adults
More than 2,000 adult subjects with HIV-1 infection have been treated with EMTRIVA alone or in combination with other antiretroviral agents for periods of 10 days to 200 weeks in clinical trials.
The most common adverse reactions (incidence greater than or equal to 10%, any severity) identified from any of the three large, controlled clinical trials include headache, diarrhea, nausea, fatigue, dizziness, depression, insomnia, abnormal dreams, rash, abdominal pain, asthenia, increased cough, and rhinitis.
In Trials 301A and 303, the most common adverse reactions that occurred in subjects receiving EMTRIVA with other antiretroviral agents were headache, diarrhea, nausea, and rash, which were generally mild to moderate. Approximately 1% of subjects discontinued participation in the clinical trials due to these events. All adverse reactions were reported with similar frequency in EMTRIVA and control treatment groups except for skin discoloration, which was reported with higher frequency in the EMTRIVA-treated group.
Skin discoloration, manifested by hyperpigmentation on the palms or soles, was generally mild and asymptomatic. The mechanism and clinical significance are unknown.
A summary of EMTRIVA treatment-emergent clinical adverse reactions in Trials 301A and 303 is provided in Table 2.
Table 2 Selected Treatment-Emergent Adverse Reactions (All Grades, Regardless of Causality) Reported in ≥3% of EMTRIVA-Treated Subjects in Either Trial 301A or 303 (0–48 Weeks) 303 301A EMTRIVA + AZT/d4T + NNRTI/PI
(N=294)3TC + AZT/d4T + NNRTI/PI
(N=146)EMTRIVA + didanosine + EFV
(N=286)d4T + didanosine + EFV
(N=285)AZT=zidovudine; d4T=stavudine; NNRTI/PI=non-nucleoside reverse transcriptase inhibitor/protease inhibitor; 3TC=lamivudine; EFV=efavirenz. - * Rash event includes rash, pruritus, maculopapular rash, urticaria, vesiculobullous rash, pustular rash, and allergic reaction.
Body as a Whole Asthenia 16% 10% 12% 17% Headache 13% 6% 22% 25% Abdominal pain 8% 11% 14% 17% Digestive System Diarrhea 23% 18% 23% 32% Nausea 18% 12% 13% 23% Vomiting 9% 7% 9% 12% Dyspepsia 4% 5% 8% 12% Musculoskeletal Myalgia 4% 4% 6% 3% Arthralgia 3% 4% 5% 6% Nervous System Insomnia 7% 3% 16% 21% Depressive disorders 6% 10% 9% 13% Paresthesia 5% 7% 6% 12% Dizziness 4% 5% 25% 26% Neuropathy/peripheral neuritis 4% 3% 4% 13% Abnormal dreams 2% <1% 11% 19% Respiratory Rhinitis 18% 12% 12% 10% Increased cough 14% 11% 14% 8% Skin Rash event* 17% 14% 30% 33% Laboratory Abnormalities: Laboratory abnormalities in these trials occurred with similar frequency in the EMTRIVA and comparator groups. A summary of Grades 3−4 laboratory abnormalities is provided in Table 3.
Table 3 Treatment-Emergent Grades 3–4 Laboratory Abnormalities Reported in ≥1% of EMTRIVA-Treated Subjects in Either Trial 301A or 303 303 301A EMTRIVA + AZT/d4T + NNRTI/PI
(N=294)3TC + AZT/d4T + NNRTI/PI
(N=146)EMTRIVA + Didanosine + EFV
(N=286)d4T + Didanosine + EFV
(N=285)- * ULN = Upper limit of normal
Any ≥ Grade 3 Laboratory Abnormality 31% 28% 34% 38% ALT (>5.0 × ULN*) 2% 1% 5% 6% AST (>5.0 × ULN) 3% <1% 6% 9% Bilirubin (>2.5 × ULN) 1% 2% <1% <1% Creatine kinase
(>4.0 × ULN)11% 14% 12% 11% Neutrophils (<750 mm3) 5% 3% 5% 7% Pancreatic amylase
(>2.0 × ULN)2% 2% <1% 1% Serum amylase
(>2.0 × ULN)2% 2% 5% 10% Serum glucose
<40 or >250 mg/dL)3% 3% 2% 3% Serum lipase
(>2.0 × ULN)<1% <1% 1% 2% Triglycerides
(>750 mg/dL)10% 8% 9% 6% In Trial 934, 511 antiretroviral-naïve subjects received efavirenz (EFV) administered in combination with either EMTRIVA + tenofovir disoproxil fumarate (TDF) (N=257) or AZT/3TC (N=254) for 144 weeks. The most common adverse reactions (incidence greater than or equal to 10%, all grades) included diarrhea, nausea, fatigue, headache, dizziness, depression, insomnia, abnormal dreams, and rash. Table 4 provides the treatment-emergent adverse reactions (Grades 2−4) occurring in greater than or equal to 5% of subjects treated in any treatment group.
Table 4 Selected Adverse Reactions* (Grades 2–4) Reported in ≥5% in Any Treatment Group in Trial 934 (0–144 Weeks) EMTRIVA + TDF + EFV† AZT/3TC + EFV N=257 N=254 - * Frequencies of adverse reactions are based on all treatment-emergent adverse events, regardless of relationship to study drug.
- † From Weeks 96 to 144 of the trial, subjects received TRUVADA® with EFV in place of EMTRIVA + TDF with EFV.
- ‡ Rash event includes rash, exfoliative rash, rash generalized, rash macular, rash maculo-papular, rash pruritic, and rash vesicular.
Fatigue 9% 8% Depression 9% 7% Nausea 9% 7% Diarrhea 9% 5% Dizziness 8% 7% Upper respiratory tract infections 8% 5% Sinusitis 8% 4% Rash event‡ 7% 9% Headache 6% 5% Insomnia 5% 7% Nasopharyngitis 5% 3% Vomiting 2% 5% Laboratory Abnormalities: Laboratory abnormalities observed in Trial 934 were generally consistent with those seen in previous trials (Table 5).
Table 5 Significant Laboratory Abnormalities Reported in ≥1% of Subjects in Any Treatment Group in Trial 934 (0–144 Weeks) EMTRIVA + TDF + EFV* AZT/3TC + EFV N=257 N=254 - * From Weeks 96 to 144 of the trial, subjects received TRUVADA with EFV in place of EMTRIVA + TDF with EFV.
Any ≥ Grade 3 Laboratory Abnormality 30% 26% Fasting Cholesterol (>240 mg/dL) 22% 24% Creatine Kinase
(M: >990 U/L)
(F: >845 U/L)9% 7% Serum Amylase (>175 U/L) 8% 4% Alkaline Phosphatase (>550 U/L) 1% 0% AST
(M: >180 U/L)
(F: >170 U/L)3% 3% ALT
(M: >215 U/L)
(F: >170 U/L)2% 3% Hemoglobin (<8.0 mg/dL) 0% 4% Hyperglycemia (>250 mg/dL) 2% 1% Hematuria (>75 RBC/HPF) 3% 2% Glycosuria (3+) <1% 1% Neutrophils (<750/mm3) 3% 5% Fasting Triglycerides (>750 mg/dL) 4% 2% Adverse Reactions from Clinical Trials Experience in Pediatric Subjects
Assessment of adverse reactions in pediatric subjects is based on data from Trial 203, an open label, uncontrolled trial of 116 HIV-1 infected subjects who received FTC through 48 weeks. The adverse reaction profile in pediatric subjects was generally comparable to that observed in clinical trials of EMTRIVA in adult subjects [see Adverse Reactions (6.1)]. Hyperpigmentation was more frequent in children. Additional adverse reactions identified from this trial include anemia.
Selected treatment-emergent adverse events, regardless of causality, reported in subjects during 48 weeks of treatment were the following: infection (44%), hyperpigmentation (32%), increased cough (28%), vomiting (23%), otitis media (23%), rash (21%), rhinitis (20%), diarrhea (20%), fever (18%), pneumonia (15%), gastroenteritis (11%), abdominal pain (10%), and anemia (7%). Treatment-emergent Grades 3−4 laboratory abnormalities were experienced by 9% of pediatric subjects, including elevated amylase (>2.0 × ULN) (n=4), decreased neutrophils (<750/mm3) (n=3), elevated ALT (>5 × ULN) (n=2), elevated CPK (>4 × ULN) (n=2) and one subject each with elevated bilirubin (>3.0 × ULN), elevated GGT (>10 × ULN), elevated lipase (>2.5 × ULN), decreased hemoglobin (<7 g/dL), and decreased glucose (<40 mg/dL).
-
7 DRUG INTERACTIONS
The potential for drug interactions with EMTRIVA has been studied in combination with AZT, indinavir, d4T, famciclovir, and tenofovir DF (TDF). There were no clinically significant drug interactions for any of these drugs. Drug interactions trials are described elsewhere in the labeling [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to EMTRIVA during pregnancy. Healthcare providers are encouraged to register patients by calling the Antiretroviral Pregnancy Registry (APR) at 1-800-258-4263.
Risk Summary
Available data from the APR show no increase in the overall risk of major birth defects with first trimester exposure for emtricitabine (FTC) (2.3%) compared with the background rate for major birth defects of 2.7% in a U.S. reference population of the Metropolitan Atlanta Congenital Defects Program (MACDP) (see Data). The rate of miscarriage for individual drugs is not reported in the APR. In the U.S. general population, the estimated background risk of miscarriage in clinically recognized pregnancies is 15–20%.
In animal reproduction studies, no adverse developmental effects were observed when FTC was administered at exposures ≥60 times that of the recommended daily dose of EMTRIVA (see Data).
Data
Human Data
Based on prospective reports to the APR of exposures to FTC-containing regimens during pregnancy resulting in live births (including over 2,700 exposed in the first trimester and over 1,200 exposed in the second/third trimester), there was no increase between FTC and overall birth defects compared with the background birth defect rate of 2.7% in a U.S. reference population of the MACDP. The prevalence of birth defects in live births was 2.4% (95% CI: 1.9% to 3.1%) with first trimester exposure to FTC-containing regimens and 2.3% (95% CI: 1.5% to 3.3%) with the second/third trimester exposure to FTC-containing regimens.
Prospective reports from the APR of overall major birth defects in pregnancies exposed to FTC are compared with a U.S. background major birth defect rate. Methodologic limitations of the APR include the use of MACDP as the external comparator group. Limitations of using an external comparator include differences in methodology and populations, as well as confounding due to the underlying disease.
Additionally, published observational studies on FTC exposure in pregnancy have not shown an increased risk for major malformations.
Animal Data
FTC was administered orally to pregnant mice (at 0, 250, 500, or 1,000 mg/kg/day), and rabbits (at 0, 100, 300, or 1,000 mg/kg/day) through organogenesis (on gestation days 6 through 15, and 7 through 19, respectively). No significant toxicological effects were observed in embryo-fetal toxicity studies performed with FTC in mice at exposures (AUC) approximately 60 times higher and in rabbits at approximately 120 times higher than human exposures at the recommended daily dose. In a pre/postnatal development study in mice, FTC was administered orally at doses up to 1,000 mg/kg/day; no significant adverse effects directly related to drug were observed in the offspring exposed daily from before birth (in utero) through sexual maturity at daily exposures (AUC) of approximately 60 times higher than human exposures at the recommended daily dose.
8.2 Lactation
Risk Summary
The Centers for Disease Control and Prevention recommend that HIV-1 infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV-1.
Based on published data, FTC has been shown to be present in human breast milk. It is not known if FTC affects milk production or has effects on the breastfed child.
Because of the potential for: (1) HIV transmission (in HIV-negative infants); (2) developing viral resistance (in HIV-positive infants); and (3) adverse reactions in a breastfed infant similar to those seen in adults, instruct mothers not to breastfeed if they are taking EMTRIVA.
8.4 Pediatric Use
The safety and efficacy of FTC in patients between 3 months and 21 years of age is supported by data from three open-label, nonrandomized clinical trials in which FTC was administered to 169 HIV-1 infected treatment-naïve and experienced (defined as virologically suppressed on a 3TC containing regimen for which FTC was substituted for 3TC) subjects [see Clinical Studies (14.4)].
The pharmacokinetics of FTC were studied in 20 neonates born to HIV-1 positive mothers [see Clinical Studies (14.4)]. All neonates were HIV-1 negative at the end of the trial; the efficacy of FTC in preventing or treating HIV-1 could not be determined.
8.5 Geriatric Use
Clinical trials of EMTRIVA did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger subjects. In general, dose selection for the elderly patient should be cautious, keeping in mind the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Renal Impairment
Modify the dose or dosing interval for EMTRIVA in patients with creatinine clearance below 50 mL/min or in patients with end stage renal disease requiring dialysis [see Dosage and Administration (2.6)].
-
10 OVERDOSAGE
If overdose occurs, the patient should be monitored for evidence of toxicity, and standard supportive treatment applied as necessary.
Hemodialysis treatment removes approximately 30% of the FTC dose over a 3-hour dialysis period starting within 1.5 hours of FTC dosing (blood flow rate of 400 mL/min and a dialysate flow rate of 600 mL/min). It is not known whether FTC can be removed by peritoneal dialysis.
-
11 DESCRIPTION
EMTRIVA is the brand name of emtricitabine (FTC), a synthetic nucleoside analog with activity against human immunodeficiency virus type 1 (HIV-1) reverse transcriptase.
The chemical name of FTC is 5-fluoro-1-(2R,5S)-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Emtricitabine is the (-) enantiomer of a thio analog of cytidine, which differs from other cytidine analogs in that it has a fluorine in the 5-position.
It has a molecular formula of C8H10FN3O3S and a molecular weight of 247.24. It has the following structural formula:
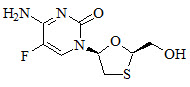
Emtricitabine is a white to off-white powder with a solubility of approximately 112 mg/mL in water at 25 °C. The partition coefficient (log P) for FTC is −0.43 and the pKa is 2.65.
EMTRIVA is available as capsules or as an oral solution.
EMTRIVA capsules are for oral administration. Each capsule contains 200 mg of FTC and the inactive ingredients crospovidone, magnesium stearate, microcrystalline cellulose, povidone, titanium dioxide, gelatin, and FD&C blue No. 2.
EMTRIVA oral solution is for oral administration. One milliliter (1 mL) of EMTRIVA oral solution contains 10 mg of FTC in an aqueous solution with the following inactive ingredients: cotton candy flavor, FD&C yellow No. 6, edetate disodium, methylparaben and propylparaben (added as preservatives), sodium phosphate (monobasic), propylene glycol, water, and xylitol (added as a sweetener). Sodium hydroxide and hydrochloric acid may be used to adjust pH.
-
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
Adults
The pharmacokinetic properties of FTC were evaluated in healthy subjects and HIV-1-infected subjects. Emtricitabine pharmacokinetics are similar between these populations.
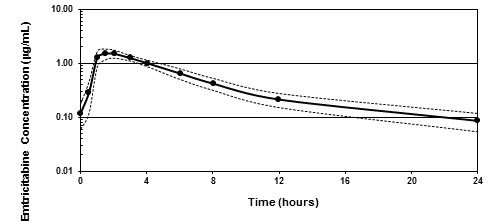
Figure 1 shows the mean steady-state plasma FTC concentration-time profile in 20 HIV-1-infected subjects receiving EMTRIVA capsules.
Figure 1 Mean (± 95% CI) Steady-State Plasma FTC Concentrations in HIV-1 Infected Adults (N=20)
Absorption
Emtricitabine is rapidly and extensively absorbed following oral administration, with peak plasma concentrations occurring at 1–2 hours postdose. Following multiple dose oral administration of EMTRIVA capsules to 20 HIV-1 infected subjects, the (mean ± SD) steady-state plasma FTC peak concentration (Cmax) was 1.8 ± 0.7 µg/mL and the area-under the plasma concentration-time curve over a 24-hour dosing interval (AUC) was 10.0 ± 3.1 µg∙hr/mL. The mean steady-state plasma trough concentration at 24 hours postdose was 0.09 µg/mL. The mean absolute bioavailability of EMTRIVA capsules was 93%, while the mean absolute bioavailability of EMTRIVA oral solution was 75%. The relative bioavailability of EMTRIVA oral solution was approximately 80% of EMTRIVA capsules.
The multiple dose pharmacokinetics of FTC are dose proportional over a dose range of 25–200 mg.
Distribution
In vitro binding of FTC to human plasma proteins was less than 4% and independent of concentration over the range of 0.02–200 µg/mL. At peak plasma concentration, the mean plasma to blood drug concentration ratio was ~1.0 and the mean semen to plasma drug concentration ratio was ~4.0.
Metabolism
Following administration of radiolabelled FTC, complete recovery of the dose was achieved in urine (~86%) and feces (~14%). Thirteen percent (13%) of the dose was recovered in urine as three putative metabolites. The biotransformation of FTC includes oxidation of the thiol moiety to form the 3'-sulfoxide diastereomers (~9% of dose) and conjugation with glucuronic acid to form 2'-O-glucuronide (~4% of dose). No other metabolites were identifiable.
Elimination
The plasma FTC half-life is approximately 10 hours. The renal clearance of FTC is greater than the estimated creatinine clearance, suggesting elimination by both glomerular filtration and active tubular secretion. There may be competition for elimination with other compounds that are also renally eliminated.
Effects of Food on Oral Absorption
EMTRIVA capsules and oral solution may be taken with or without food. Emtricitabine systemic exposure (AUC) was unaffected while Cmax decreased by 29% when EMTRIVA capsules were administered with food (an approximately 1000 kcal high-fat meal). Emtricitabine systemic exposure (AUC) and Cmax were unaffected when 200 mg EMTRIVA oral solution was administered with either a high-fat or low-fat meal.
Specific Populations
Geriatric Patients
The pharmacokinetics of FTC have not been fully evaluated in the elderly (65 years of age and older).
Pediatric Patients
The pharmacokinetics of FTC at steady state were determined in 77 HIV-1 infected pediatric subjects, and the pharmacokinetic profile was characterized in four age groups (Table 6). The FTC exposure achieved in pediatric subjects receiving a daily dose of 6 mg/kg up to a maximum of 240 mg oral solution or a 200-mg capsule is similar to exposures achieved in adult subjects receiving a once-daily dose of 200 mg.
The pharmacokinetics of FTC were studied in 20 neonates born to HIV-1 positive mothers. Each mother received prenatal and intrapartum combination antiretroviral therapy. Neonates received up to 6 weeks of AZT prophylactically after birth. The neonates were administered two short courses of FTC oral solution (each 3 mg/kg once daily × 4 days) during the first 3 months of life. The AUC observed in neonates who received a daily dose of 3 mg/kg of FTC was similar to the AUC observed in pediatric subjects aged 3 months to 17 years who received a daily dose of FTC as a 6 mg/kg oral solution up to 240 mg or as a 200-mg capsule (Table 6).
Table 6 Mean ± SD Pharmacokinetic Parameters by Age Groups for Pediatric Subjects and Neonates Receiving EMTRIVA Capsules or Oral Solution HIV-1-exposed Neonates HIV-1 Infected Pediatric Subjects Age 0–3 mo
(N=20)*3–24 mo
(N=14)25 mo–6 yr
(N=19)7–12yr
(N=17)13–17 yr
(N=27)- * Two pharmacokinetic evaluations were conducted in 20 neonates over the first 3 months of life. Median (range) age of infant on day of pharmacokinetic evaluation was 26 (5–81) days.
- † Mean (range).
Formulation Capsule (n) 0 0 0 10 26 Oral Solution (n) 20 14 19 7 1 Dose (mg/kg)† 3.1 (2.9–3.4) 6.1 (5.5–6.8) 6.1 (5.6–6.7) 5.6 (3.1–6.6) 4.4 (1.8–7.0) Cmax (µg/mL) 1.6 ± 0.6 1.9 ± 0.6 1.9 ± 0.7 2.7 ± 0.8 2.7 ± 0.9 AUC (µg∙hr/mL) 11.0 ± 4.2 8.7 ± 3.2 9.0 ± 3.0 12.6 ± 3.5 12.6 ± 5.4 T1/2 (hr) 12.1 ± 3.1 8.9 ± 3.2 11.3 ± 6.4 8.2 ± 3.2 8.9 ± 3.3 Patients with Renal Impairment
The pharmacokinetics of FTC are altered in subjects with renal impairment [see Warnings and Precautions (5.4)]. In adult subjects with creatinine clearance below 50 mL/min or with end-stage renal disease (ESRD) requiring dialysis, Cmax and AUC of FTC were increased (Table 7). The effects of renal impairment on FTC pharmacokinetics in pediatric patients are not known.
Table 7 Pharmacokinetic Parameters (Mean ± SD) of FTC in Adult Subjects with Varying Degrees of Renal Function Creatinine Clearance (mL/min) >80
(N=6)50–80
(N=6)30–49
(N=6)<30
(N=5)ESRD*
<30
(N=5)- * ESRD subjects requiring dialysis
- † NA = Not Applicable
Baseline creatinine clearance (mL/min) 107 ± 21 59.8 ± 6.5 40.9 ± 5.1 22.9 ± 5.3 8.8 ± 1.4 Cmax (µg/mL) 2.2 ± 0.6 3.8 ± 0.9 3.2 ± 0.6 2.8 ± 0.7 2.8 ± 0.5 AUC (µg∙hr/mL) 11.8 ± 2.9 19.9 ± 1.2 25.1 ± 5.7 33.7± 2.1 53.2 ± 9.9 CL/F (mL/min) 302 ± 94 168 ± 10 138 ± 28 99 ± 6 64 ± 12 CLr (mL/min) 213 ± 89 121 ± 39 69 ± 32 30 ± 11 NA† Assessment of Drug Interactions
At concentrations up to 14-fold higher than those observed in vivo, FTC did not inhibit in vitro drug metabolism mediated by any of the following human CYP isoforms: CYP1A2, CYP2A6, CYP2B6, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. FTC did not inhibit the enzyme responsible for glucuronidation (uridine-5'-disphosphoglucuronyl transferase). Based on the results of these in vitro experiments and the known elimination pathways of FTC, the potential for CYP-mediated interactions involving FTC with other medicinal products is low.
EMTRIVA has been evaluated in healthy volunteers in combination with TDF, AZT, indinavir, famciclovir, and d4T. Tables 8 and 9 summarize the pharmacokinetic effects of coadministered drug on FTC pharmacokinetics and effects of FTC on the pharmacokinetics of coadministered drug.
Table 8 Drug Interactions: Change in Pharmacokinetic Parameters for FTC in the Presence of the Coadministered Drug* Coadministered Drug Dose of Coadministered Drug (mg) FTC Dose (mg) N % Change of FTC Pharmacokinetic Parameters† (90% CI) Cmax AUC Cmin - * All interaction trials conducted in healthy volunteers.
- † ↑ = Increase; ⇔ = No Effect; NA = Not Applicable
Famciclovir 500 × 1 200 × 1 12 ⇔ ⇔ NA Indinavir 800 × 1 200 × 1 12 ⇔ ⇔ NA Stavudine 40 × 1 200 × 1 6 ⇔ ⇔ NA Tenofovir DF 300 once daily × 7 days 200 once daily × 7 days 17 ⇔ ⇔ ↑ 20
(↑ 12 to ↑ 29)Zidovudine 300 twice daily × 7 days 200 once daily × 7 days 27 ⇔ ⇔ ⇔ Table 9 Drug Interactions: Change in Pharmacokinetic Parameters for Coadministered Drug in the Presence of FTC* Coadministered Drug Dose of Coadministered Drug (mg) FTC Dose (mg) N % Change of Coadministered Drug Pharmacokinetic Parameters† (90% CI) Cmax AUC Cmin - * All interaction trials conducted in healthy volunteers.
- † ↑ = Increase; ⇔ = No Effect; NA = Not Applicable
Famciclovir 500 × 1 200 × 1 12 ⇔ ⇔ NA Indinavir 800 × 1 200 × 1 12 ⇔ ⇔ NA Stavudine 40 × 1 200 × 1 6 ⇔ ⇔ NA Tenofovir DF 300 once daily × 7 days 200 once daily × 7 days 17 ⇔ ⇔ ⇔ Zidovudine 300 twice daily × 7 days 200 once daily × 7 days 27 ↑ 17
(↑ 0 to ↑ 38)↑ 13
(↑ 5 to↑ 20)⇔ 12.4 Microbiology
Mechanism of Action
FTC, a synthetic nucleoside analog of cytidine, is phosphorylated by cellular enzymes to form emtricitabine 5'-triphosphate (FTC-TP), which inhibits the activity of the HIV-1 reverse transcriptase (RT) by competing with the natural substrate deoxycytidine 5'-triphosphate and by being incorporated into nascent viral DNA resulting in chain termination. FTC-TP is a weak inhibitor of mammalian DNA polymerases α, β, ε, and mitochondrial DNA polymerase γ.
Antiviral Activity
The antiviral activity of FTC against laboratory and clinical isolates of HIV-1 was assessed in lymphoblastoid cell lines, the MAGI-CCR5 cell line, and peripheral blood mononuclear cells. The 50% effective concentration (EC50) values for FTC were in the range of 0.0013–0.64 µM (0.0003–0.158 µg/mL). The median EC50, range, and number of isolates for the laboratory isolates were 0.07, 0.009−0.62, 10; 0.011, 0.002−0.03, 12; 0.055, 0.0015−0.09, 4 respectively for lymphoblastoid, PBMC, and reporter cells. The median EC50, range, and number of isolates for the clinical isolates were 0.05, 0.0105−0.64, 15; 0.015, 0.004−0.028, 6 respectively for lymphoblastoid cells and PBMCs. No antagonism was observed in drug combination studies of emtricitabine with nucleoside reverse transcriptase inhibitors (NRTIs) (abacavir, lamivudine, stavudine, tenofovir, zidovudine), non-nucleoside reverse transcriptase inhibitors (NNRTIs) (delavirdine, efavirenz, nevirapine), and protease inhibitors (amprenavir, nelfinavir, ritonavir, saquinavir). FTC displayed antiviral activity in cell culture against HIV-1 clades A, B, C, D, E, F, and G (EC50 values ranged from 0.007–0.075 µM) and showed strain-specific activity against HIV-2 (EC50 values ranged from 0.007–1.5 µM in PBMCs and MAGI cells).
Resistance
FTC-resistant isolates of HIV-1 have been selected in cell culture and in vivo. Genotypic analysis of these isolates showed that the reduced susceptibility to FTC was associated with a valine or isoleucine (M184V/I) substitution in the HIV-1 RT.
FTC-resistant isolates of HIV-1 have been recovered from some subjects treated with FTC alone or in combination with other antiretroviral agents. In a clinical trial of treatment-naïve subjects treated with EMTRIVA, didanosine, and efavirenz (EFV) [see Clinical Studies (14.2)], viral isolates from 37.5% of subjects with virologic failure showed reduced susceptibility to FTC. Genotypic analysis of these isolates showed that the resistance was due to M184V/I substitutions in the HIV-1 RT.
In a clinical trial of treatment-naïve subjects treated with either EMTRIVA, TDF, and EFV or AZT/3TC and EFV [see Clinical Studies (14.2)], resistance analysis was performed on HIV-1 isolates from all confirmed virologic failure subjects with greater than 400 copies/mL of HIV-1 RNA at Week 144 or early discontinuation. Development of EFV resistance-associated substitutions occurred most frequently and was similar between the treatment arms. The M184V amino acid substitution, associated with resistance to EMTRIVA and 3TC, was observed in 2/19 analyzed subject isolates in the EMTRIVA + TDF group and in 10/29 analyzed subject isolates in the AZT/3TC group. Through 144 weeks of Trial 934, no subjects have developed a detectable K65R substitution in their HIV-1 as analyzed through standard genotypic analysis.
Cross Resistance
Cross-resistance among certain NRTIs has been recognized. FTC-resistant isolates (M184V/I) were cross-resistant to 3TC but retained susceptibility in cell culture to AZT, didanosine, d4T, and tenofovir, and to NNRTIs (delavirdine, EFV, and nevirapine). HIV-1 isolates containing the K65R substitution, selected in vivo by abacavir, didanosine, and tenofovir, demonstrated reduced susceptibility to inhibition by FTC. Viruses harboring substitutions conferring reduced susceptibility to AZT and d4T (M41L, D67N, K70R, L210W, T215Y/F, K219Q/E) or didanosine (L74V) remained sensitive to FTC. HIV-1 containing the K103N substitution associated with resistance to NNRTIs was susceptible to FTC.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In long-term oral carcinogenicity studies of FTC, no drug-related increases in tumor incidence were found in mice at doses up to 750 mg/kg/day (26 times the human systemic exposure at the therapeutic dose of 200 mg/day) or in rats at doses up to 600 mg/kg/day (31 times the human systemic exposure at the therapeutic dose).
Emtricitabine was not genotoxic in the reverse mutation bacterial test (Ames test) or mouse lymphoma or mouse micronucleus assays.
Emtricitabine did not affect fertility in male rats at approximately 140-fold or in male and female mice at approximately 60-fold higher exposures (AUC) than in humans given the recommended 200 mg daily dose. Fertility was normal in the offspring of mice exposed daily from before birth (in utero) through sexual maturity at daily exposures (AUC) of approximately 60-fold higher than human exposures at the recommended 200 mg daily dose.
-
14 CLINICAL STUDIES
14.1 Overview of Clinical Trials
The efficacy and safety of EMTRIVA were evaluated in the trials summarized in Table 10.
Table 10 Trials Conducted with EMTRIVA in Adult and Pediatric Subjects Trial Population Trial Arms (N)* Timepoint
(Weeks)- * Randomized and dosed.
- † Randomized, open-label, active-controlled trial.
- ‡ Randomized, double-blind, active-controlled trial.
- § Nonrandomized, open-label trial.
Trial 934†
(NCT00112047)HIV-1 treatment-naïve adults EMTRIVA+TDF+EFV (227)
AZT/3TC+EFV (229)144 Trial 301A‡
(NCT00006208)EMTRIVA+didanosine+EFV (286)
d4T+didanosine+EFV (285)48 Trial 303†
(NCT00002416)HIV-1 treatment-experienced adults EMTRIVA+AZT/d4T+NNRTI/PI (294)
3TC+AZT/d4T+NNRTI/PI (146)48 Trial 202§
(NCT00016718)HIV-1 treatment-naïve and experienced pediatrics EMTRIVA+didanosine+EFV (43) 48 Trial 203§
(NCT00743340)EMTRIVA+d4T+lopinavir/ritonavir (116) 48 Trial 211§
(NCT00642291)EMTRIVA+didanosine+EFV (16) 48 14.2 Clinical Trial Results in Treatment-Naïve Adults
Trial 934
Data through 144 weeks are reported for Trial 934, a randomized, open-label, active-controlled multicenter clinical trial comparing EMTRIVA + TDF administered in combination with EFV versus AZT/3TC fixed-dose combination administered in combination with EFV in 511 antiretroviral-naïve subjects. From Weeks 96 to 144 of the trial, subjects received FTC/TDF fixed-dose combination with EFV in place of EMTRIVA + TDF with EFV. Subjects had a mean age of 38 years (range 18–80); 86% were male, 59% were Caucasian, and 23% were Black. The mean baseline CD4+ cell count was 245 cells/mm3 (range 2–1191) and median baseline plasma HIV-1 RNA was 5.01 log10 copies/mL (range 3.56–6.54). Subjects were stratified by baseline CD4+ cell count (< or ≥200 cells/mm3); 41% had CD4+ cell counts <200 cells/mm3 and 51% of subjects had baseline viral loads >100,000 copies/mL. Table 11 provides treatment outcomes through 48 and 144 weeks for those subjects who did not have EFV resistance at baseline.
Table 11 Virologic Outcomes of Randomized Treatment at Week 48 and 144 (Trial 934) Outcomes Week 48 Week 144 EMTRIVA+TDF+EFV
(N=244)AZT/3TC+EFV
(N=243)EMTRIVA+TDF+EFV
(N=227)*AZT/3TC+EFV
(N=229)*- * Subjects who were responders at Week 48 or Week 96 (HIV-1 RNA <400 copies/mL) but did not consent to continue in the trial after Week 48 or Week 96 were excluded from analysis.
- † Subjects achieved and maintained confirmed HIV-1 RNA <400 copies/mL through Weeks 48 and 144.
- ‡ Includes confirmed viral rebound and failure to achieve confirmed <400 copies/mL through Weeks 48 and 144.
- § Includes lost to follow-up, subject withdrawal, noncompliance, protocol violation and other reasons.
Responder† 84% 73% 71% 58% Virologic failure‡ 2% 4% 3% 6% Rebound 1% 3% 2% 5% Never suppressed 0% 0% 0% 0% Change in antiretroviral regimen 1% 1% 1% 1% Death <1% 1% 1% 1% Discontinued due to adverse event 4% 9% 5% 12% Discontinued for other reasons§ 10% 14% 20% 22% Through Week 48, 84%, and 73% of subjects in the EMTRIVA + TDF group and the AZT/3TC group, respectively, achieved and maintained HIV-1 RNA <400 copies/mL (71% and 58% through Week 144). The difference in the proportion of subjects who achieved and maintained HIV-1 RNA <400 copies/mL through 48 weeks largely results from the higher number of discontinuations due to adverse events and other reasons in the AZT/3TC group in this open-label trial. In addition, 80% and 70% of subjects in the EMTRIVA + TDF group and the AZT/3TC group, respectively, achieved and maintained HIV-1 RNA <50 copies/mL through Week 48 (64% and 56% through Week 144). The mean increase from baseline in CD4+ cell count was 190 cells/mm3 in the EMTRIVA + TDF group and 158 cells/mm3 in the AZT/3TC group at Week 48 (312 and 271 cells/mm3 at Week 144).
Through 48 weeks, 7 subjects in the EMTRIVA + TDF group and 5 subjects in the AZT/3TC group experienced a new CDC Class C event (10 and 6 subjects through 144 weeks).
Trial 301A
Trial 301A was a 48-week double-blind, active-controlled, multicenter clinical trial comparing EMTRIVA (200 mg once daily) administered in combination with didanosine and EFV versus d4T, didanosine, and EFV in 571 antiretroviral naïve adult subjects. Subjects had a mean age of 36 years (range 18–69); 85% were male, 52% Caucasian, 16% African-American, and 26% Hispanic. Subjects had a mean baseline CD4+ cell count of 318 cells/mm3 (range 5–1317) and a median baseline plasma HIV-1 RNA of 4.9 log10 copies/mL (range 2.6–7.0). Thirty-eight percent of subjects had baseline viral loads >100,000 copies/mL and 31% had CD4+ cell counts <200 cells/mL. Table 12 provides treatment outcomes through 48 weeks.
Table 12 Virologic Outcomes of Randomized Treatment at Week 48 (Trial 301A) Outcomes EMTRIVA+ Didanosine+ EFV
(N=286)d4T+ Didanosine+ EFV
(N=285)- * Subjects achieved and maintained confirmed HIV RNA <400 copies/mL (<50 copies/mL) through Week 48.
- † Includes subjects who failed to achieve virologic suppression or rebounded after achieving virologic suppression.
- ‡ Includes lost to follow-up, subject withdrawal, non-compliance, protocol violation, and other reasons.
Responder* 81% (78%) 68% (59%) Virologic Failure† 3% 11% Death 0% <1% Discontinuation Due to Adverse Event 7% 13% Discontinuation for Other Reasons‡ 9% 8% The mean increase from baseline in CD4+ cell count was 168 cells/mm3 for the EMTRIVA arm and 134 cells/mm3 for the d4T arm.
Through 48 weeks, 5 subjects (1.7%) in the EMTRIVA group experienced a new CDC Class C event compared to 7 subjects (2.5%) in the d4T group.
14.3 Clinical Trial Results in Treatment-Experienced Adults
Trial 303
Trial 303 was a 48-week, open-label, active-controlled, multicenter clinical trial comparing EMTRIVA (200 mg once daily) to 3TC, in combination with d4T or AZT and a protease inhibitor or NNRTI in 440 adult subjects who were on a 3TC-containing triple-antiretroviral drug regimen for at least 12 weeks prior to trial entry and had HIV-1 RNA ≤400 copies/mL.
Subjects were randomized 1:2 to continue therapy with 3TC (150 mg twice daily) or to switch to EMTRIVA (200 mg once daily). All subjects were maintained on their stable background regimen. Subjects had a mean age of 42 years (range 22–80); 86% were male, 64% Caucasian, 21% African-American, and 13% Hispanic. Subjects had a mean baseline CD4+ cell count of 527 cells/mm3 (range 37–1909), and a median baseline plasma HIV-1 RNA of 1.7 log10 copies/mL (range 1.7–4.0).
The median duration of prior antiretroviral therapy was 27.6 months. Table 13 provides treatment outcomes through 48 weeks.
Table 13 Virologic Outcomes of Randomized Treatment at Week 48 (Trial 303) Outcomes EMTRIVA+ AZT/d4T+ NNRTI/PI
(N=294)3TC+ AZT/d4T+ NNRTI/PI
(N=146)- * Subjects achieved and maintained confirmed HIV RNA <400 copies/mL (<50 copies/mL) through Week 48.
- † Includes subjects who failed to achieve virologic suppression or rebounded after achieving virologic suppression.
- ‡ Includes lost to follow-up, subject withdrawal, non-compliance, protocol violation, and other reasons.
Responder* 77% (67%) 82% (72%) Virologic Failure† 7% 8% Death 0% <1% Discontinuation Due to Adverse Event 4% 0% Discontinuation for Other Reasons‡ 12% 10% The mean increase from baseline in CD4+ cell count was 29 cells/mm3 for the EMTRIVA arm and 61 cells/mm3 for the 3TC arm.
Through 48 weeks, in the EMTRIVA group 2 subjects (0.7%) experienced a new CDC Class C event compared to 2 subjects (1.4%) in the 3TC group.
14.4 Clinical Trial Results in Pediatrics
In three open-label, nonrandomized clinical trials, FTC was administered to 169 HIV-1 infected treatment-naïve and experienced (defined as virologically suppressed on a 3TC-containing regimen for which FTC was substituted for 3TC) subjects between 3 months and 21 years of age. Subjects received once-daily EMTRIVA oral solution (6 mg/kg to a maximum of 240 mg/day) or EMTRIVA capsules (a single 200 mg capsule once daily) in combination with at least two other antiretroviral agents.
Subjects had a mean age of 7.9 years (range 0.3–21); 49% were male, 15% Caucasian, 61% Black, and 24% Hispanic. Subjects had a median baseline HIV-1 RNA of 4.6 log10 copies/mL (range 1.7–6.4) and a mean baseline CD4+ cell count of 745 cells/mm3 (range 2–2650). Through 48 weeks of therapy, the overall proportion of subjects who achieved and sustained an HIV-1 RNA <400 copies/mL was 86%, and <50 copies/mL was 73%. The mean increase from baseline in CD4+ cell count was 232 cells/mm3 (−945, +1512). The adverse reaction profile observed during these clinical trials was similar to that of adult subjects, with the exception of the occurrence of anemia and higher frequency of hyperpigmentation in children [see Adverse Reactions (6.1)].
The pharmacokinetics of FTC were studied in 20 neonates born to HIV-1 positive mothers. Each mother received prenatal and intrapartum combination antiretroviral therapy. Neonates received up to 6 weeks of AZT prophylactically after birth. The neonates were administered two short courses of FTC oral solution (each 3 mg/kg once daily × 4 days) during the first 3 months of life. FTC exposures in neonates were similar to the exposures achieved in subjects aged 3 months to 17 years [see Clinical Pharmacology (12.3)]. During the two short dosing periods on FTC, there were no safety issues identified in the treated neonates. All neonates were HIV-1 negative at the end of the trial; the efficacy of FTC in preventing or treating HIV-1 could not be determined.
-
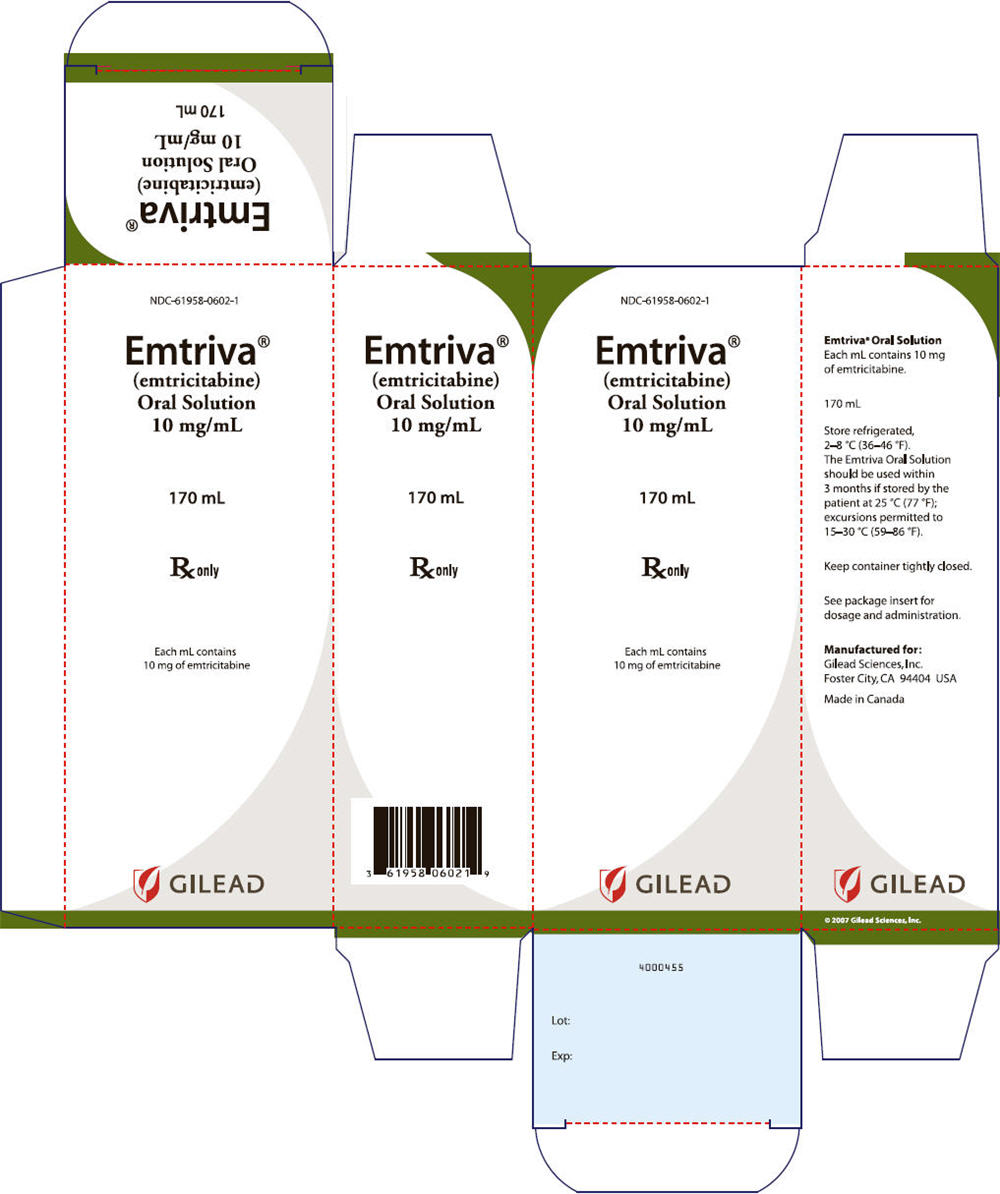
16 HOW SUPPLIED/STORAGE AND HANDLING
EMTRIVA capsules are available in bottles containing 30 capsules with child-resistant closure as follows:
200 mg of FTC capsules are size 1 hard gelatin capsules with a blue cap and white body and printed with "200 mg" in black on the cap and with "GILEAD" and the corporate logo in black on the body (NDC 61958–0601–1).
- Store EMTRIVA capsules at 25 °C (77 °F); excursions permitted to 15–30 °C (59–86 °F).
- Dispense only in original container. Keep container tightly closed.
EMTRIVA oral solution is a clear, orange to dark orange liquid containing 10 mg/mL of FTC and is available in unit of use plastic, amber bottles containing 170 mL of oral solution closed with a child resistant closure, and packaged with a marked dosing cup (NDC 61958–0602–1).
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Severe Acute Exacerbation of Hepatitis B in Patients Coinfected with HIV-1 and HBV
Inform patients that severe acute exacerbations of hepatitis B have been reported in patients who are coinfected with HIV-1 and hepatitis B virus (HBV) who have discontinued EMTRIVA. Advise patients not to discontinue EMTRIVA without first informing their healthcare provider. All patients should be tested for HBV infection before or when starting EMTRIVA and those who are infected with HBV need close medical follow-up for several months after stopping EMTRIVA to monitor for exacerbations of hepatitis [see Warnings and Precautions (5.1)].
Immune Reconstitution Syndrome
Inform patients that in some patients with advanced HIV infection (AIDS), signs and symptoms of inflammation from previous infections may occur soon after anti-HIV treatment is started. It is believed that these symptoms are due to an improvement in the body's immune response, enabling the body to fight infections that may have been present with no obvious symptoms. Advise patients to inform their healthcare provider immediately of any symptoms of infection [see Warnings and Precautions (5.2)].
Lactic Acidosis and Severe Hepatomegaly
Inform patients that lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported. Treatment with EMTRIVA should be suspended in any patient who develops clinical symptoms suggestive of lactic acidosis or pronounced hepatotoxicity [see Warnings and Precautions (5.3)].
Pregnancy Registry
Inform patients that there is an antiretroviral pregnancy registry to monitor fetal outcomes of pregnant women exposed to EMTRIVA [see Use in Specific Populations (8.1)].
Lactation
Instruct mothers not to breastfeed if they are taking EMTRIVA because of the risk of passing the HIV-1 virus to the baby [see Use in Specific Populations (8.2)].
-
SPL UNCLASSIFIED SECTION
© 2018 Gilead Sciences, Inc. All rights reserved.
Manufactured for and distributed by:
Gilead Sciences, Inc.
Foster City, CA 94404EMTRIVA and TRUVADA are trademarks of Gilead Sciences, Inc., or its related companies. All other trademarks referenced herein are the property of their respective owners.
21-500-896-GS-019
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: December/2018 Patient Information
EMTRIVA® (em-treev'-ah)
(emtricitabine)
capsules and oral solutionWhat is the most important information I should know about EMTRIVA?
EMTRIVA can cause serious side effects, including:
-
Worsening of Hepatitis B virus infection (HBV). Your healthcare provider will test you for HBV before starting treatment with EMTRIVA. If you have HBV infection and take EMTRIVA, your HBV may get worse (flare-up) if you stop taking EMTRIVA. A "flare-up" is when your HBV infection suddenly returns in a worse way than before.
- Do not stop taking EMTRIVA without first talking to your healthcare provider.
- Do not run out of EMTRIVA. Refill your prescription or talk to your healthcare provider before your EMTRIVA is all gone.
- If you stop taking EMTRIVA, your healthcare provider will need to check your health often and do blood tests regularly for several months to check your HBV infection, or give you a medication to treat hepatitis B. Tell your healthcare provider about any new or unusual symptoms you may have after you stop taking EMTRIVA.
For more information about side effects, see the section "What are the possible side effects of EMTRIVA?"
What is EMTRIVA?
EMTRIVA is a prescription medicine used in combination with other antiretroviral medicines to treat Human Immunodeficiency Virus-1 (HIV-1) infection.
HIV-1 is the virus that causes Acquired Immune Deficiency Syndrome (AIDS).
Who should not take EMTRIVA?
Do not take EMTRIVA if you are allergic to emtricitabine or any of the ingredients in EMTRIVA. See the end of this leaflet for a complete list of ingredients in EMTRIVA.
What should I tell my healthcare provider before taking EMTRIVA?
Before taking EMTRIVA, tell your healthcare provider about all of your medical conditions, including if you:
- have liver problems, including HBV infection
- have kidney problems or receive kidney dialysis treatment
- are pregnant or planning to become pregnant. It is not known if EMTRIVA can harm your unborn baby. Tell your healthcare provider if you become pregnant during treatment with EMTRIVA.
Pregnancy Registry: There is a pregnancy registry for women who take EMTRIVA during pregnancy. The purpose of this registry is to collect information about the health of you and your baby. Talk to your healthcare provider about how you can take part in this registry. - are breastfeeding or plan to breastfeed. EMTRIVA can pass to your baby in your breast milk. Do not breastfeed if you have HIV-1 because of the risk of passing HIV-1 to your baby. Talk with your healthcare provider about the best way to feed your baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Some medicines may interact with EMTRIVA. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.
You can ask your healthcare provider or pharmacist for a list of medicines that interact with EMTRIVA. Do not start a new medicine without telling your healthcare provider. Your healthcare provider can tell you if it is safe to take EMTRIVA with other medicines.
How should I take EMTRIVA?
- Take EMTRIVA exactly as your healthcare provider tells you to take it.
- Dosing in adults: Take EMTRIVA by mouth 1 time each day.
- Dosing in children: Take EMTRIVA by mouth 1 time each day. The child's healthcare provider will calculate the right dose of EMTRIVA (oral solution or capsule) based on the child's weight.
- EMTRIVA may be taken with or without food.
- Do not miss a dose of EMTRIVA. Missing a dose lowers the amount of medicine in your blood. Refill your EMTRIVA prescription before you run out of medicine.
- Do not change your dose or stop taking EMTRIVA without first talking with your healthcare provider. Stay under a healthcare provider's care when taking EMTRIVA.
- If you take too much EMTRIVA, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of EMTRIVA?
EMTRIVA may cause serious side effects, including:
- See "What is the most important information I should know about EMTRIVA?"
- Changes in your immune system (Immune Reconstitution Syndrome) can happen when an HIV-infected person starts taking antiretroviral medicines. Your immune system may get stronger and begin to fight infections that have been hidden in your body for a long time. Tell your healthcare provider right away if you start having any new symptoms after starting EMTRIVA.
- Too much lactic acid in your blood (lactic acidosis). Too much lactic acid is a serious but rare medical emergency that can lead to death. Tell your healthcare provider right away if you develop any of these symptoms:
- weakness or being more tired than usual
- being short of breath or fast breathing
- cold or blue hands and feet
- fast or abnormal heartbeat
- unusual muscle pain
- stomach pain with nausea and vomiting
- feel dizzy or lightheaded
- Severe liver problems. In rare cases, severe liver problems can happen that can lead to death. Your healthcare provider may tell you to stop taking EMTRIVA if you develop new or worse liver problems during treatment with EMTRIVA. Tell your healthcare provider right away if you develop any of these symptoms:
- fatigue
- weakness
- yellowing of your skin or the white part of your eyes (jaundice)
- light colored stools
- nausea and vomiting
- confusion
- dark "tea-colored" urine
- loss of appetite for several days or longer
- stomach-area (abdominal) swelling and pain
The most common side effects of EMTRIVA include:
- headache
- diarrhea
- nausea
- tiredness
- dizziness
- depression
- problems sleeping
- abnormal dreams
- rash
- abdominal pain
- weakness
- cough
- runny nose
Skin discoloration in children may also happen with EMTRIVA.
These are not all the possible side effects of EMTRIVA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store EMTRIVA?
- Store EMTRIVA capsules at room temperature between 68°F to 77°F (20°C to 25°C) .
- Store EMTRIVA oral solution in the refrigerator between 36°F to 46°F (2°C to 8°C). Do not freeze.
- You may also store EMTRIVA oral solution at room temperature between 68°F to 77°F (20°C to 25°C) for up to 3 months. Throw away any unused EMTRIVA oral solution after 3 months.
- Keep EMTRIVA in its original container and keep the container tightly closed.
- Do not use EMTRIVA if seal over bottle opening is broken or missing.
Keep EMTRIVA and all other medicines out of reach of children.
General information about the safe and effective use of EMTRIVA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use EMTRIVA for a condition for which it was not prescribed. Do not give EMTRIVA to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about EMTRIVA that is written for health professionals.
What are the ingredients of EMTRIVA?
Active Ingredient: emtricitabine
Inactive Ingredients:
EMTRIVA capsules: crospovidone, magnesium stearate, microcrystalline cellulose, povidone, titanium dioxide, gelatin, and FD&C blue No. 2.
EMTRIVA oral solution: cotton candy flavor, FD&C yellow No. 6, edetate disodium, methylparaben and propylparaben, sodium phosphate (monobasic), propylene glycol, water, and xylitol. Sodium hydroxide and hydrochloric acid may be used to adjust pH.
Manufactured for and distributed by:
Gilead Sciences, Inc. Foster City, CA 94404
EMTRIVA is a trademark of Gilead Sciences, Inc., or its related companies. © 2018 Gilead Sciences, Inc. All rights reserved.
For more information, call 1-800-GILEAD-5 (1-800-445-3235).
21-500-896-GS-019 -
Worsening of Hepatitis B virus infection (HBV). Your healthcare provider will test you for HBV before starting treatment with EMTRIVA. If you have HBV infection and take EMTRIVA, your HBV may get worse (flare-up) if you stop taking EMTRIVA. A "flare-up" is when your HBV infection suddenly returns in a worse way than before.
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - Representative Bottle Label
NDC: 61958-0601-1
Emtriva®
(emtricitabine)
Capsules, 200 mg30 capsules
Rx only
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EMTRIVA
emtricitabine capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 61958-0601 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EMTRICITABINE (UNII: G70B4ETF4S) (EMTRICITABINE - UNII:G70B4ETF4S) EMTRICITABINE 200 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSPOVIDONE (15 MPA.S AT 5%) (UNII: 68401960MK) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) MAGNESIUM STEARATE (UNII: 70097M6I30) WATER (UNII: 059QF0KO0R) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) Product Characteristics Color WHITE (white opaque body with light-blue cap) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code 200mg;GILEAD Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 61958-0601-1 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/02/2003 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021500 07/02/2003 EMTRIVA
emtricitabine solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 61958-0602 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EMTRICITABINE (UNII: G70B4ETF4S) (EMTRICITABINE - UNII:G70B4ETF4S) EMTRICITABINE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYDROCHLORIC ACID (UNII: QTT17582CB) METHYLPARABEN (UNII: A2I8C7HI9T) SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) XYLITOL (UNII: VCQ006KQ1E) Product Characteristics Color ORANGE (Clear, orange to dark orange) Score Shape Size Flavor COTTON CANDY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 61958-0602-1 1 in 1 CARTON 09/28/2005 1 170 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021896 09/28/2005 Labeler - Gilead Sciences, Inc. (185049848)
Trademark Results [Emtriva]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EMTRIVA 78239710 2852092 Live/Registered |
Gilead Sciences, Inc. 2003-04-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.