NIGHTTIME SLEEP AID- diphenhydramine hcl 25 mg tablet, coated
Nighttime Sleep Aid by
Drug Labeling and Warnings
Nighttime Sleep Aid by is a Otc medication manufactured, distributed, or labeled by Health Pharma USA LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
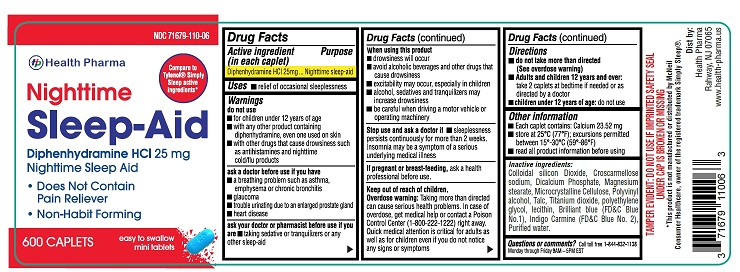
- Active ingredient (in each caplet)
- Purpose
- Uses:
-
Warnings
do not use:
- for children under 12 years of age
- with any other product containing diphenhydramine, even one used on skin
- with other drugs that cause drowsiness such as antihistamines and nighttime cold/flu products
ask a doctor before use if you have
- a breathing problem such as asthma, emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
-
heart disease
ask your doctor or pharmacist before use if you are
- taking sedative or tranquilizers or any other sleep-aid
When using this product
- drowsiness will occur
- avoid alcoholic beverages and other drugs that cause drowsiness
- excitability may occur, especially in children
- alcohol, sedatives and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
sleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of a serious underlying medical illness.
Keep out of reach of children.
Overdose warning: Taking more than directed can cause serious health problems. In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away. Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms
- Directions
- Other Information
- Inactive Ingredients
- Questions or comments?
- Principal Display Panel-300 count
- Principal Display Panel-600 count
- Principal Display Panel-1000 count
-
INGREDIENTS AND APPEARANCE
NIGHTTIME SLEEP AID
diphenhydramine hcl 25 mg tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 71679-110 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) WATER (UNII: 059QF0KO0R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TALC (UNII: 7SEV7J4R1U) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) Product Characteristics Color blue Score no score Shape CAPSULE Size 11mm Flavor Imprint Code S25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71679-110-03 300 in 1 BOTTLE; Type 0: Not a Combination Product 06/18/2025 2 NDC: 71679-110-06 600 in 1 BOTTLE; Type 0: Not a Combination Product 06/18/2025 3 NDC: 71679-110-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 06/18/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M010 06/18/2025 Labeler - Health Pharma USA LLC (080804485) Registrant - Health Pharma USA LLC (080804485) Establishment Name Address ID/FEI Business Operations ELYSIUM PHARMACEUTICALS LIMITED 915664486 manufacture(71679-110) Establishment Name Address ID/FEI Business Operations HHH PHARMA USA LLC 062788820 pack(71679-110)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.