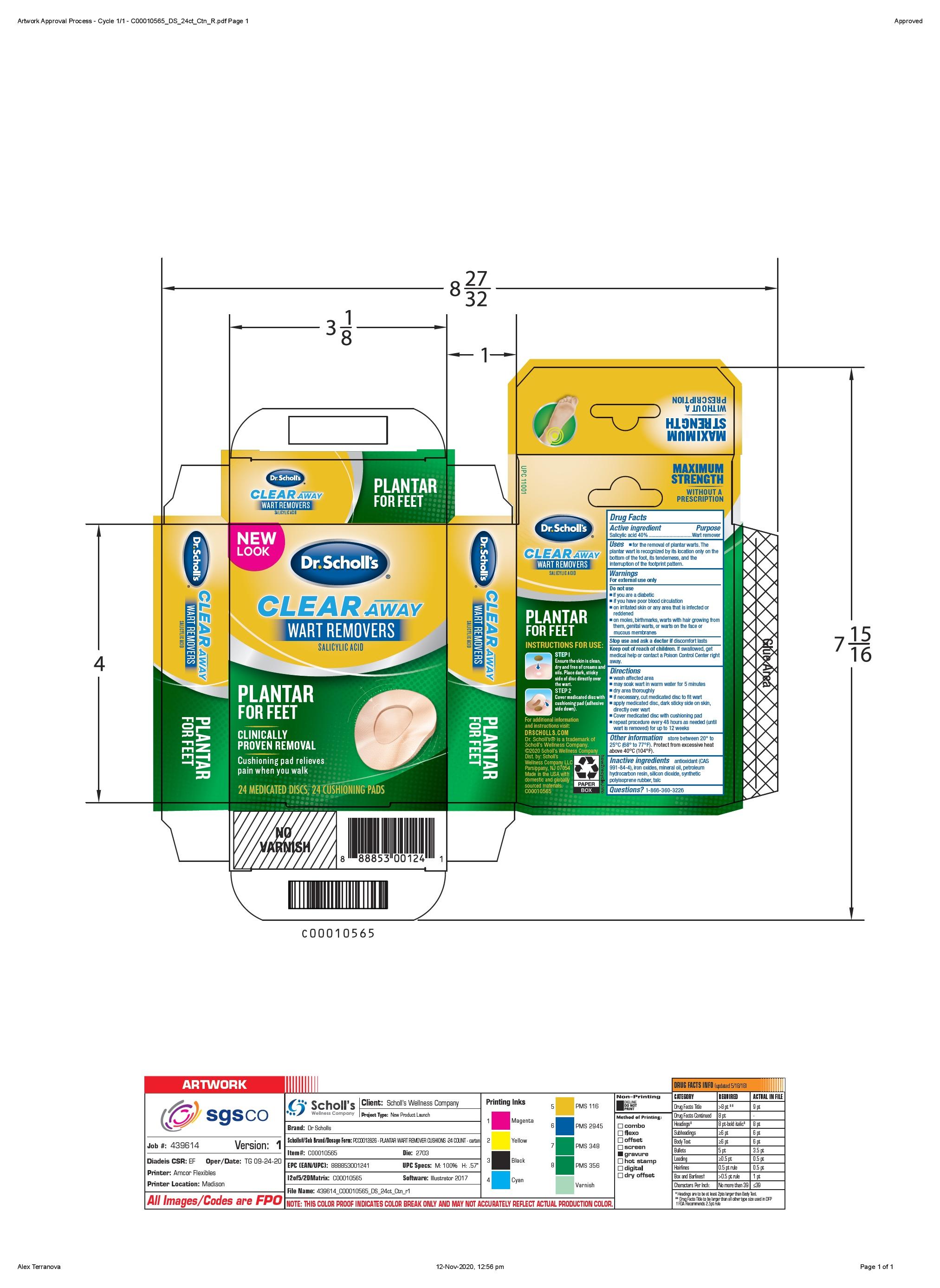
Clear Away Wart Removers (Scapa)
Scholls Wellness Company LLC by
Drug Labeling and Warnings
Scholls Wellness Company LLC by is a Otc medication manufactured, distributed, or labeled by Scapa Tapes North America LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SCHOLLS WELLNESS COMPANY LLC CLEAR AWAY WART REMOVERS- salicylic acid disc
Scapa Tapes North America LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Clear Away Wart Removers (Scapa)
Uses
Uses
for the removal of plantar warts. The plantar wart is recognized by its location only on teh bottom of the foot, its tenderness, and the interuption of the footprint pattern.
Do not use
if you are a diabetic
if you have poor blood circulation
on irritated skin or any area that is infected or reddened
on moles, birthmarks, warts with hair growing from them, genital warts, or warts on the face or mucous membranes.
Keep out of reach of children. If swallowed,
get medical help or contact a Poison Control Center right away.
Directions
Directions
wash affected area
may soak wart in warm water for 5 minutes
dry area thoroughly
if necessary, cut medicated disc to fit wart
apply medicated disc, dark sticky side on skin, directly over wart
cover medicated disc with enclosed cushion
repeat procedure every 48 hours as needed (until wart is removed) for up to 12 weeks
Other information
Other information
store between 20ºC to 25ºC (68º to 77ºF). Protect from excessive heat (temperatures above 40ºC (104ºF)
| SCHOLLS WELLNESS COMPANY LLC
CLEAR AWAY WART REMOVERS
salicylic acid disc |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Scapa Tapes North America LLC (079995435) |
| Registrant - Scapa Tapes North America LLC (079995435) |