MIRALAX- polyethylene glycol 3350 powder, for solution
MiraLAX by
Drug Labeling and Warnings
MiraLAX by is a Otc medication manufactured, distributed, or labeled by Bayer HealthCare LLC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each dose) (Bottle Only)
- Purpose
- Active ingredient (in each dose) (Packet Only)
- Purpose
- Use
-
Warnings
Ask a doctor before use if you have
- nausea, vomiting or abdominal pain
- a sudden change in bowel habits that lasts over 2 weeks
- irritable bowel syndrome
-
Directions (Bottle Only)
- do not take more than directed unless advised by your doctor
- the bottle top is a measuring cap marked to contain 17 grams of powder when filled to the indicated line (white section in cap)
- adults and children 17 years of age and older:
- fill to top of white section in cap which is marked to indicate the correct dose (17 g)
- stir and dissolve in any 4 to 8 ounces of beverage (cold, hot or room temperature) then drink
- use once a day
- use no more than 7 days
- children 16 years of age or under: ask a doctor
-
Directions (Packet Only)
- do not take more than directed unless advised by your doctor
- adults and children 17 years of age and older:
- stir and dissolve one packet of powder (17 g) in any 4 to 8 ounces of beverage (cold, hot or room temperature) then drink
- use once a day
- use no more than 7 days
- children 16 years of age or under: ask a doctor
- Other information
- Inactive ingredients
- Questions or comments?
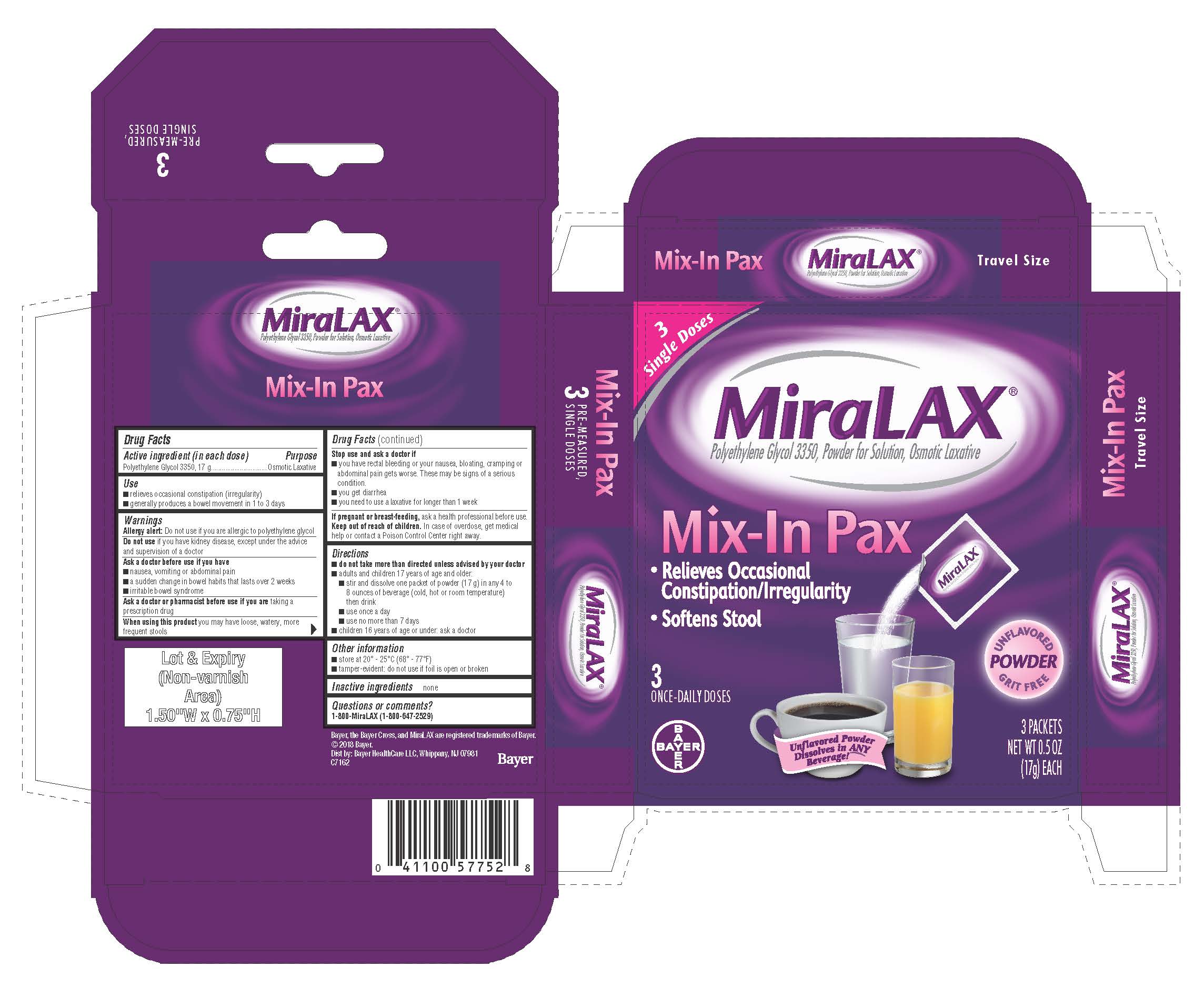
- PRINCIPAL DISPLAY PANEL - 17 g × 3 Packet Carton
- PRINCIPAL DISPLAY PANEL - 510 g Bottle Label
- PRINCIPAL DISPLAY PANEL - 17 g × 10 Packet Carton
- PRINCIPAL DISPLAY PANEL - Twinpack 34 ounce daily doses
-
INGREDIENTS AND APPEARANCE
MIRALAX
polyethylene glycol 3350 powder, for solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 11523-7268 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) (POLYETHYLENE GLYCOL 3350 - UNII:G2M7P15E5P) POLYETHYLENE GLYCOL 3350 17 g in 17 g Product Characteristics Color white (Colorless upon dissolution) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 11523-7268-3 10 in 1 CARTON 02/01/2007 1 17 g in 1 PACKET; Type 0: Not a Combination Product 2 NDC: 11523-7268-8 24 in 1 CARTON 02/01/2007 2 17 g in 1 PACKET; Type 0: Not a Combination Product 3 NDC: 11523-7268-7 5 in 1 CARTON 02/01/2007 01/01/2015 3 17 g in 1 PACKET; Type 0: Not a Combination Product 4 NDC: 11523-7268-4 765 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/01/2007 5 NDC: 11523-7268-9 20 in 1 CARTON 02/01/2007 5 17 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022015 02/01/2007 MIRALAX
polyethylene glycol 3350 powder, for solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 11523-7234 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) (POLYETHYLENE GLYCOL 3350 - UNII:G2M7P15E5P) POLYETHYLENE GLYCOL 3350 17 g in 17 g Product Characteristics Color white (Colorless upon dissolution) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 11523-7234-1 17 g in 1 PACKET; Type 0: Not a Combination Product 02/01/2007 2 NDC: 11523-7234-2 119 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/01/2007 3 NDC: 11523-7234-3 238 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/01/2007 4 NDC: 11523-7234-4 510 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/01/2007 5 NDC: 11523-7234-5 12 in 1 CARTON 02/01/2007 01/01/2012 5 17 g in 1 PACKET; Type 0: Not a Combination Product 6 NDC: 11523-7234-6 17 g in 1 PACKET; Type 0: Not a Combination Product 02/01/2007 7 NDC: 11523-7234-9 2 in 1 CELLO PACK 02/01/2007 01/01/2014 7 510 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022015 02/01/2007 MIRALAX
polyethylene glycol 3350 powder, for solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 11523-7341 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) (POLYETHYLENE GLYCOL 3350 - UNII:G2M7P15E5P) POLYETHYLENE GLYCOL 3350 17 g in 17 g Product Characteristics Color white (Colorless upon dissolution) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 11523-7341-1 612 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/01/2007 2 NDC: 11523-7341-2 3 in 1 CARTON 02/01/2007 2 17 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022015 02/01/2007 MIRALAX
polyethylene glycol 3350 powder, for solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 11523-4357 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) (POLYETHYLENE GLYCOL 3350 - UNII:G2M7P15E5P) POLYETHYLENE GLYCOL 3350 17 g in 17 g Product Characteristics Color white (colorless upon dissolution) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 11523-4357-1 2 in 1 PACKAGE, COMBINATION 02/01/2007 01/01/2018 1 765 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC: 11523-4357-3 2 in 1 PACKAGE, COMBINATION 02/01/2007 2 578 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 3 NDC: 11523-4357-5 24 in 1 CARTON 05/01/2018 3 17 g in 1 PACKET; Type 0: Not a Combination Product 4 NDC: 11523-4357-2 2 in 1 CARTON 02/01/2007 4 20 in 1 CARTON 4 17 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022015 02/01/2007 Labeler - Bayer HealthCare LLC. (112117283)
Trademark Results [MiraLAX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MIRALAX 85746766 4426594 Live/Registered |
BAYER HEALTHCARE LLC 2012-10-05 |
 MIRALAX 78686831 3120379 Live/Registered |
Bayer HealthCare LLC 2005-08-05 |
 MIRALAX 77753736 3735255 Dead/Cancelled |
BAYER CONSUMER CARE HOLDINGS LLC 2009-06-05 |
 MIRALAX 77753735 3735254 Dead/Cancelled |
BAYER CONSUMER CARE HOLDINGS LLC 2009-06-05 |
 MIRALAX 77702783 not registered Dead/Abandoned |
MSD Consumer Care, Inc. 2009-03-31 |
 MIRALAX 77097551 3412356 Dead/Cancelled |
BAYER CONSUMER CARE HOLDINGS LLC 2007-02-02 |
 MIRALAX 75622252 2982512 Dead/Cancelled |
SCHERING-PLOUGH HEALTHCARE PRODUCTS, INC. 1999-01-15 |
 MIRALAX 75109262 not registered Dead/Abandoned |
Braintree Laboratories, Inc. 1996-05-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.