EPURIS- isotretinoin capsule
Epuris by
Drug Labeling and Warnings
Epuris by is a Prescription medication manufactured, distributed, or labeled by Galephar Pharmaceutical Research Inc., Galephar Pharmaceutical Research Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
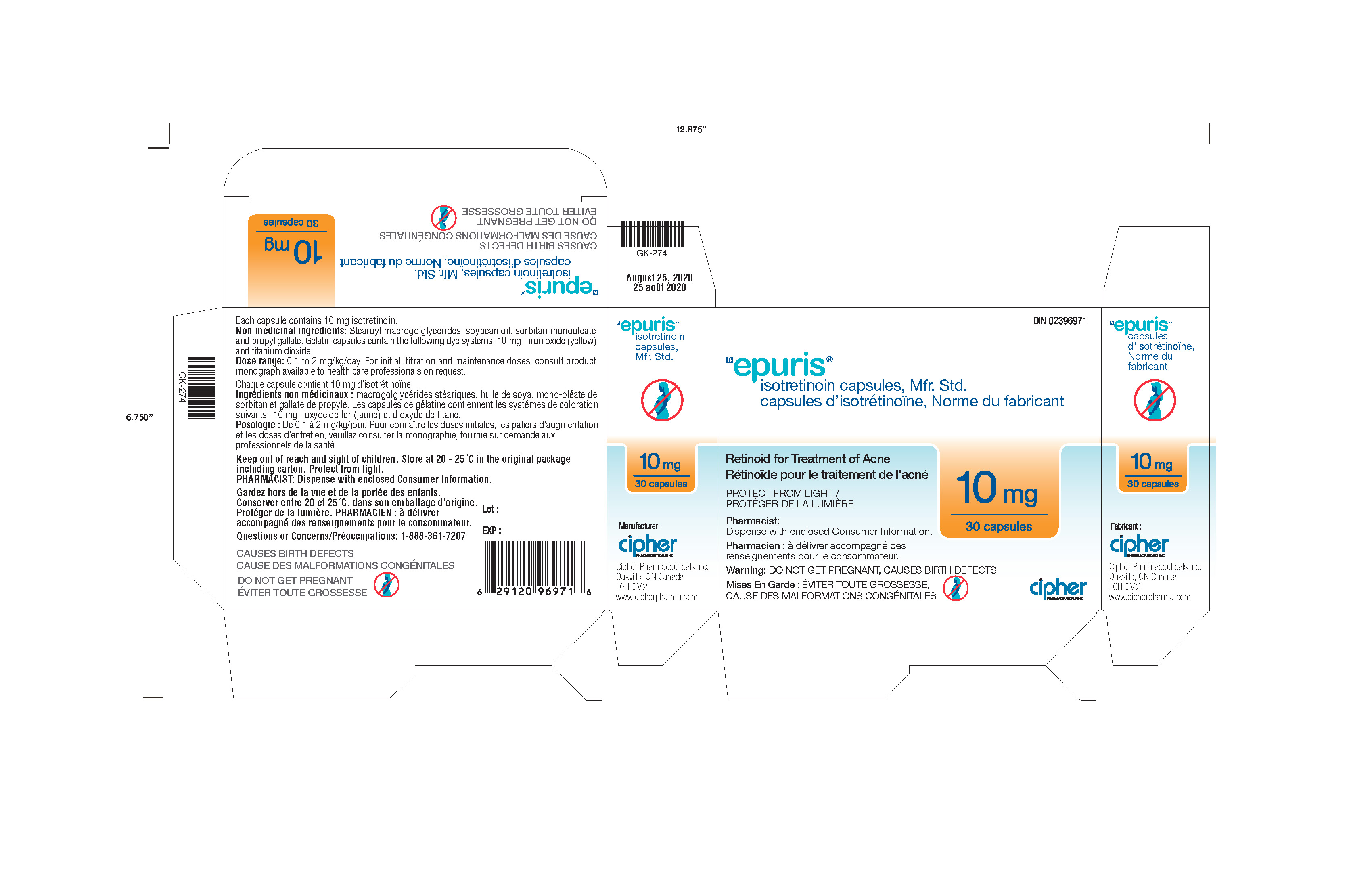
- Epuris 10 mg Box of 3 blister cards
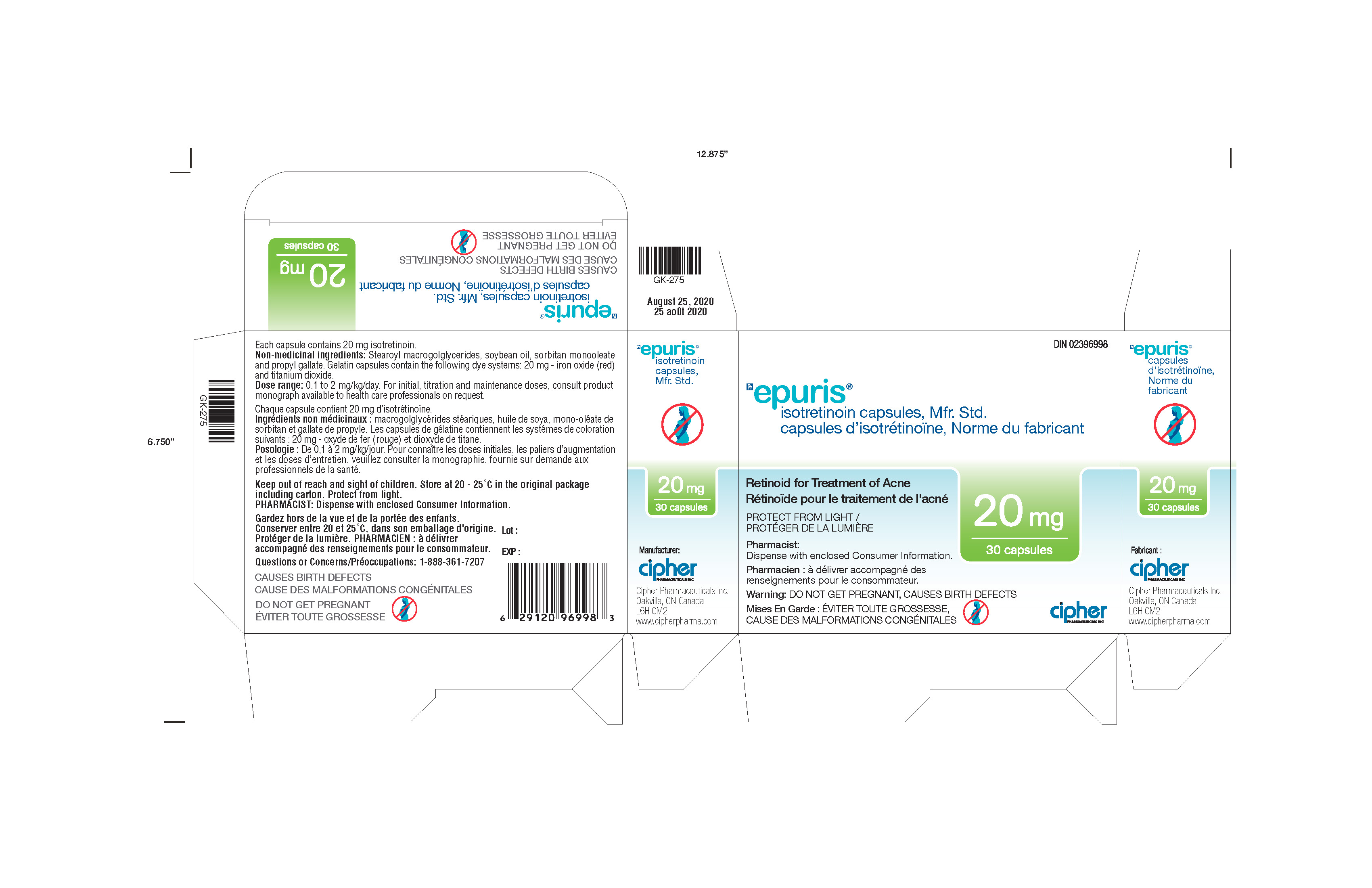
- Epuris 20 mg Box of 3 blister cards
- Epuris 30 mg Box of 3 blister cards
- Epuris 40 mg Box of 3 blister cards
-
INGREDIENTS AND APPEARANCE
EPURIS
isotretinoin capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66277-339 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOTRETINOIN (UNII: EH28UP18IF) (ISOTRETINOIN - UNII:EH28UP18IF) ISOTRETINOIN 30 mg Inactive Ingredients Ingredient Name Strength PROPYL GALLATE (UNII: 8D4SNN7V92) SOYBEAN OIL (UNII: 241ATL177A) PEG-32 HYDROGENATED PALM GLYCERIDES (UNII: G6EP177239) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) Product Characteristics Color brown Score no score Shape CAPSULE Size 23mm Flavor Imprint Code G242;30 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66277-339-30 3 in 1 BOX 05/07/2013 1 NDC: 66277-339-10 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 05/07/2013 EPURIS
isotretinoin capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66277-340 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOTRETINOIN (UNII: EH28UP18IF) (ISOTRETINOIN - UNII:EH28UP18IF) ISOTRETINOIN 40 mg Inactive Ingredients Ingredient Name Strength PROPYL GALLATE (UNII: 8D4SNN7V92) SOYBEAN OIL (UNII: 241ATL177A) PEG-32 HYDROGENATED PALM GLYCERIDES (UNII: G6EP177239) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) Product Characteristics Color brown, red Score no score Shape CAPSULE Size 26mm Flavor Imprint Code G325;40 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66277-340-30 3 in 1 BOX 05/07/2013 1 NDC: 66277-340-10 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 05/07/2013 EPURIS
isotretinoin capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66277-338 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOTRETINOIN (UNII: EH28UP18IF) (ISOTRETINOIN - UNII:EH28UP18IF) ISOTRETINOIN 20 mg Inactive Ingredients Ingredient Name Strength PROPYL GALLATE (UNII: 8D4SNN7V92) SOYBEAN OIL (UNII: 241ATL177A) PEG-32 HYDROGENATED PALM GLYCERIDES (UNII: G6EP177239) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) Product Characteristics Color red Score no score Shape CAPSULE Size 22mm Flavor Imprint Code G241;20 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66277-338-30 3 in 1 BOX 05/07/2013 1 NDC: 66277-338-10 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 05/07/2013 EPURIS
isotretinoin capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66277-337 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOTRETINOIN (UNII: EH28UP18IF) (ISOTRETINOIN - UNII:EH28UP18IF) ISOTRETINOIN 10 mg Inactive Ingredients Ingredient Name Strength PROPYL GALLATE (UNII: 8D4SNN7V92) SOYBEAN OIL (UNII: 241ATL177A) PEG-32 HYDROGENATED PALM GLYCERIDES (UNII: G6EP177239) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) Product Characteristics Color yellow Score no score Shape CAPSULE Size 18mm Flavor Imprint Code G240;10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66277-337-30 3 in 1 BOX 05/07/2013 1 NDC: 66277-337-10 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 05/07/2013 Labeler - Galephar Pharmaceutical Research Inc. (003551624) Registrant - Galephar Pharmaceutical Research Inc. (968996160) Establishment Name Address ID/FEI Business Operations Galephar Pharmaceutical Research Inc 968996160 analysis(66277-337, 66277-338, 66277-339, 66277-340) , manufacture(66277-337, 66277-338, 66277-339, 66277-340) , pack(66277-337, 66277-338, 66277-339, 66277-340)
Trademark Results [Epuris]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EPURIS 98465736 not registered Live/Pending |
Atecom LLC 2024-03-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.