SELENIUM SULFIDE by Morton Grove Pharmaceuticals, Inc. SELENIUM SULFIDE lotion
SELENIUM SULFIDE by
Drug Labeling and Warnings
SELENIUM SULFIDE by is a Prescription medication manufactured, distributed, or labeled by Morton Grove Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
APPLICATION INSTRUCTIONS
Keep tightly capped. SHAKE WELL BEFORE USING. Product may damage jewelry; remove jewelry before use.
For treatment of tinea versicolor:
- Apply to affected areas and lather with a small amount of water.
- Allow to remain on skin for 10 minutes.
- Rinse body thoroughly.
- Repeat this procedure once a day for 7 days.
For treatment of dandruff and seborrheic dermatitis of the scalp.
- Massage 1 or 2 teaspoonfuls of shampoo into wet scalp.
- Allow to remain on scalp for 2 to 3 minutes.
- Rinse scalp thoroughly.
- Repeat application and rinse thoroughly.
- After treatment, wash hands well.
- Repeat treatments as directed by physician.
-
WARNINGS AND PRECAUTIONS
For External Use Only. Do not use on broken skin or inflamed areas. If allergic reactions occur, discontinue use. Avoid getting shampoo in eyes or in contact with genital area as it may cause irritation and burning.
FOR EXTERNAL USE ONLY. WARNING: KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Store at controlled room temperature, (15 - 30) °C ((59 - 86) °F) [see USP].
- CLINICAL PHARMACOLOGY
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
PRECAUTIONS
Not to be used when acute inflammation or exudation is present as increased absorption may occur.
See Warnings and Precautions section under Application Instructions.
Dermal application of 25% and 50% solutions of 2.5% selenium sulfide lotion on mice over an 88 week period, indicated no carcinogenic effects.
WHEN USED ON BODY SURFACES FOR THE TREATMENT OF TINEA VERSICOLOR, SELENIUM SULFIDE LOTION, USP 2.5% IS CLASSIFIED AS PREGNANCY CATEGORY C. Animal reproduction studies have not been conducted with selenium sulfide. It is also not known whether selenium sulfide can cause fetal harm when applied to body surfaces of a pregnant woman or can affect reproduction capacity. Under ordinary circumstances selenium sulfide should not be used for the treatment of tinea versicolor in pregnant women.
Safety and effectiveness in infants have not been established.
- ADVERSE REACTIONS
-
OVERDOSAGE
Accidental Oral Ingestion:
No documented reports of serious toxicity in humans resulting from acute ingestion of selenium sulfide, however, acute toxicity studies in animals suggest that ingestion of large amounts could result in potential human toxicity. Evacuation of the stomach contents should be considered in cases of acute oral ingestion.
- DOSAGE AND ADMINISTRATION
- For treatment of tinea versicolor
- For treatment of dandruff and seborrheic dermatitis
- HOW SUPPLIED
- WARNINGS
-
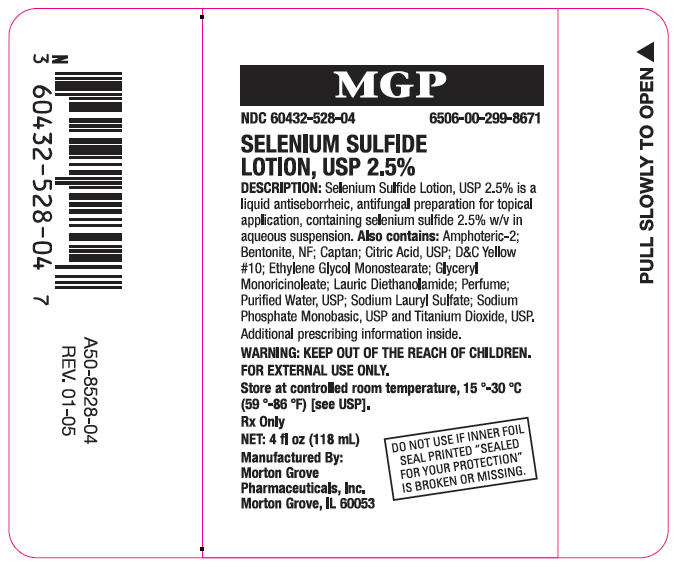
PRINCIPAL DISPLAY PANEL Bottle Label
NDC: 60432-528-04
6506-00-299-8671
SELENIUM SULFIDE
LOTION, USP 2.5%
DESCRIPTION: Selenium Sulfide Lotion, USP 2.5% is a
liquid antiseborrheic, antifungal preparation for topical
application, containing selenium sulfide 2.5% w/v in
aqueous suspension. Also contains: Amphoteric-2;
Bentonite, NF; Captan; Citric Acid, USP; D&C Yellow
#10; Ethylene Glycol Monostearate; Glyceryl
Monoricinoleate; Lauric Diethanolamide; Perfume;
Purified Water, USP; Sodium Lauryl Sulfate; Sodium
Phosphate Monobasic, USP and Titanium Dioxide, USP.
Additional prescribing information inside.
WARNING: KEEP OUT OF THE REACH OF CHILDREN.
FOR EXTERNAL USE ONLY.
Store at controlled room temperature, (15 - 30) °C
((59 - 86) °F) [see USP].
Rx Only
NET: 4 fl oz (118 mL)
Manufactured By:
Morton Grove
Pharmaceuticals, Inc.
Morton Grove, IL 60053
DO NOT USE IF INNER FOIL
SEAL PRINTED "SEALED
FOR YOUR PROTECTION"
IS BROKEN OR MISSING.
NDC: 60432-528-05
Selenium Sulfide Lotion, USP 2.5%
FOR EXTERNAL USE ONLY
Rx Only
Net: 4 fl oz (118 mL)

Selenium Sulfide Lotion, USP 2.5% Carton
-
INGREDIENTS AND APPEARANCE
SELENIUM SULFIDE
selenium sulfide lotionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 60432-528 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELENIUM SULFIDE (UNII: Z69D9E381Q) (SELENIUM SULFIDE - UNII:Z69D9E381Q) SELENIUM SULFIDE 25 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENTONITE (UNII: A3N5ZCN45C) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) SODIUM LAURYL SULFATE (UNII: 368GB5141J) DISODIUM COCOAMPHODIACETATE (UNII: 18L9G3U51M) GLYCOL STEARATE (UNII: 0324G66D0E) LAURIC DIETHANOLAMIDE (UNII: I29I2VHG38) CAPTAN (UNII: EOL5G26Q9F) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) GLYCERYL RICINOLEATE (UNII: ZUE0CEL42O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 60432-528-04 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/01/1983 2 NDC: 60432-528-05 1 in 1 CARTON 01/16/2019 2 NDC: 60432-528-04 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA088228 09/01/1983 Labeler - Morton Grove Pharmaceuticals, Inc. (801897505) Registrant - Morton Grove Pharmaceuticals, Inc. (801897505) Establishment Name Address ID/FEI Business Operations Morton Grove Pharmaceuticals, Inc. 801897505 ANALYSIS(60432-528) , MANUFACTURE(60432-528) , PACK(60432-528)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.