GUNA-IL 10- interleukin-10 solution/ drops
GUNA-IL 10 by
Drug Labeling and Warnings
GUNA-IL 10 by is a Homeopathic medication manufactured, distributed, or labeled by Guna spa. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
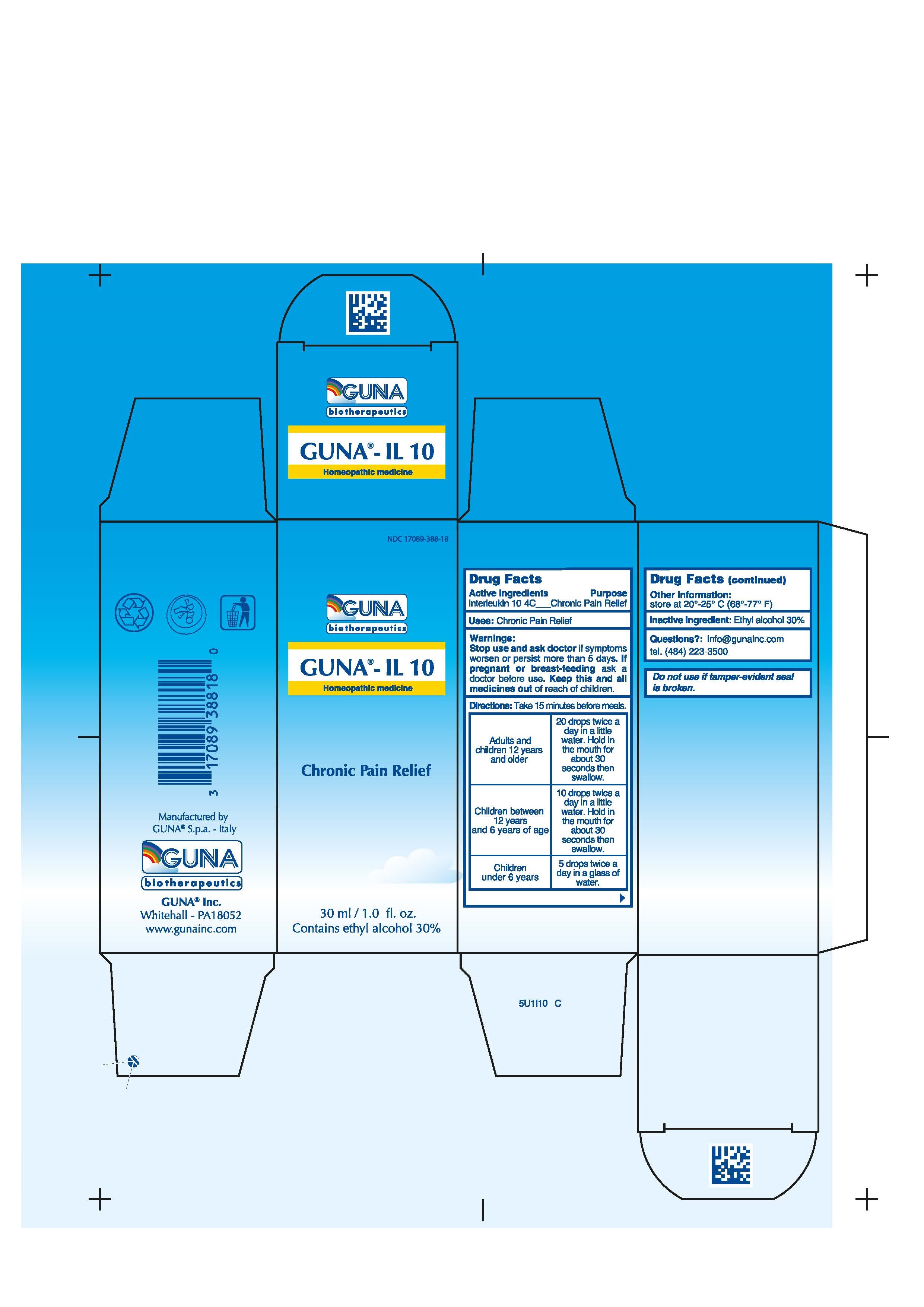
- ACTIVE INGREDIENTS/PURPOSE
- USES
- WARNINGS
- PREGNANCY
- WARNINGS
-
DIRECTIONS
Adults and children 12 years and older 20 drops twice a day in a little water. Hold in the mouth for about 30 seconds then swallow.
Children between 12 years and 6 years of age 10 drops twice a day in a little water. Hold in the mouth for about 30 seconds then swallow.
Children under 6 years 5 drops twice a day in a glass of water.
- QUESTIONS
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA-IL 10
interleukin-10 solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 17089-388 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INTERLEUKIN-10 (UNII: 9SC4O216V9) (INTERLEUKIN-10 - UNII:9SC4O216V9) INTERLEUKIN-10 4 [hp_C] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) 9 mL in 30 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17089-388-18 1 in 1 BOX 05/26/2010 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/17/2008 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-388)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.