DETROL LA- tolterodine tartrate capsule, extended release
Detrol LA by
Drug Labeling and Warnings
Detrol LA by is a Prescription medication manufactured, distributed, or labeled by Viatris Specialty LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Detrol® LA safely and effectively. See full prescribing information for Detrol® LA.
Detrol® LA (tolterodine tartrate extended release capsules), for oral administration
Initial U.S. Approval: December 2000INDICATIONS AND USAGE
DETROL LA is an antimuscarinic indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and frequency. (1)
DOSAGE AND ADMINISTRATION
- 4 mg capsules taken orally once daily with water and swallowed whole. (2.1)
- 2 mg capsules taken orally once daily with water and swallowed whole in the presence of:
- DETROL LA is not recommended for use in patients with CCr <10 mL/min. (2.2)
- DETROL LA is not recommended for use in patients with severe hepatic impairment (Child-Pugh Class C). (2.2)
DOSAGE FORMS AND STRENGTHS
Capsules: 2 mg and 4 mg (3)
CONTRAINDICATIONS
DETROL LA is contraindicated in patients with urinary retention, gastric retention, or uncontrolled narrow-angle glaucoma. DETROL LA is also contraindicated in patients with known hypersensitivity to the drug or its ingredients, or to fesoterodine fumarate extended-release tablets which, like DETROL LA, are metabolized to 5-hydroxymethyl tolterodine. (4)
WARNINGS AND PRECAUTIONS
- Anaphylaxis and angioedema requiring hospitalization and emergency medical treatment have occurred with the first or subsequent doses of DETROL LA. (5.1)
- Urinary Retention: use caution in patients with clinically significant bladder outflow obstruction because of the risk of urinary retention. (5.2)
- Gastrointestinal Disorders: use caution in patients with gastrointestinal obstructive disorders or decreased gastrointestinal motility because of the risk of gastric retention. (5.3)
- Controlled Narrow-Angle Glaucoma: use caution in patients being treated for narrow-angle glaucoma. (5.4)
- Central Nervous System Effects: Somnolence has been reported with Detrol LA. Advise patients not to drive or operate heavy machinery until they know how Detrol LA affects them (5.5).
- Myasthenia Gravis: use caution in patients with myasthenia gravis. (5.8)
- QT Prolongation: consider observations from the thorough QT study in clinical decisions to prescribe DETROL LA to patients with a known history of QT prolongation or to patients who are taking Class IA (e.g., quinidine, procainamide) or Class III (e.g., amiodarone, sotalol) antiarrhythmic medications. (5.9)
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥4% and >placebo) were dry mouth, headache, constipation, and abdominal pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Viatris at 1-877-446-3679 (1-877-4-INFO-RX) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Potent CYP3A4 Inhibitors: Coadministration may increase systemic exposure to DETROL LA. Reduce DETROL LA dose to 2 mg once daily. (7.2)
- Other Anticholinergics (antimuscarinics): Concomitant use with other anticholinergic agents may increase the frequency and/or severity of dry mouth, constipation, blurred vision, and other anticholinergic pharmacological effects. (7.6)
USE IN SPECIFIC POPULATIONS
- Renal Impairment: DETROL LA is not recommended for use in patients with CCr <10 mL/min. Dose adjustment in severe renal impairment (CCr: 10-30 mL/min). (8.6)
- Hepatic Impairment: Not recommended for use in severe hepatic impairment (Child Pugh Class C). Dose adjustment in mild to moderate hepatic impairment (Child Pugh Class A, B). (8.7)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information
2.2 Dosage Adjustment in Specific Populations
2.3 Dosage Adjustment in Presence of Concomitant Drugs
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Angioedema
5.2 Urinary Retention
5.3 Gastrointestinal Disorders
5.4 Controlled Narrow-Angle Glaucoma
5.5 Central Nervous System Effects
5.6 Hepatic Impairment
5.7 Renal Impairment
5.8 Myasthenia Gravis
5.9 Use in Patients with Congenital or Acquired QT Prolongation
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-marketing Experience
7 DRUG INTERACTIONS
7.1 Potent CYP2D6 Inhibitors
7.2 Potent CYP3A4 Inhibitors
7.3 Other Interactions
7.4 Other Drugs Metabolized by Cytochrome P450 Isoenzymes
7.5 Drug-Laboratory-Test Interactions
7.6 Other Anticholinergics
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
DETROL LA Capsules is indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and frequency [see Clinical Studies (14)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information
The recommended dose of DETROL LA Capsules is 4 mg once daily with water and swallowed whole. The dose may be lowered to 2 mg daily based on individual response and tolerability; however, limited efficacy data are available for DETROL LA 2 mg [see Clinical Studies (14)].
2.2 Dosage Adjustment in Specific Populations
For patients with mild to moderate hepatic impairment (Child-Pugh Class A or B) or severe renal impairment (CCr 10-30 mL/min), the recommended dose of DETROL LA is 2 mg once daily. DETROL LA is not recommended for use in patients with severe hepatic impairment (Child-Pugh Class C). Patients with CCr<10 mL/min have not been studied and use of DETROL LA in this population is not recommended [see Warnings and Precautions (5.6) and Use in Specific Populations (8.6, 8.7)].
2.3 Dosage Adjustment in Presence of Concomitant Drugs
For patients who are taking drugs that are potent inhibitors of CYP3A4 [e.g., ketoconazole, clarithromycin, ritonavir], the recommended dose of DETROL LA is 2 mg once daily [see Drug Interactions (7.2)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
DETROL LA is contraindicated in patients with urinary retention, gastric retention, or uncontrolled narrow-angle glaucoma. DETROL LA is also contraindicated in patients with known hypersensitivity to the drug or its ingredients, or to fesoterodine fumarate extended-release tablets which, like DETROL LA, are metabolized to 5-hydroxymethyl tolterodine [see Warnings and Precautions (5.2, 5.3, 5.4)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Angioedema
Anaphylaxis and angioedema requiring hospitalization and emergency medical treatment have occurred with the first or subsequent doses of DETROL LA. In the event of difficulty in breathing, upper airway obstruction, or fall in blood pressure, DETROL LA should be discontinued and appropriate therapy promptly provided.
5.2 Urinary Retention
Administer DETROL LA Capsules with caution to patients with clinically significant bladder outflow obstruction because of the risk of urinary retention [see Contraindications (4)].
5.3 Gastrointestinal Disorders
Administer DETROL LA with caution in patients with gastrointestinal obstructive disorders because of the risk of gastric retention.
DETROL LA, like other antimuscarinic drugs, may decrease gastrointestinal motility and should be used with caution in patients with conditions associated with decreased gastrointestinal motility (e.g., intestinal atony) [see Contraindications (4)].
5.4 Controlled Narrow-Angle Glaucoma
Administer DETROL LA with caution in patients being treated for narrow-angle glaucoma [see Contraindications (4)].
5.5 Central Nervous System Effects
Detrol LA is associated with anticholinergic central nervous system (CNS) effects [see Adverse Reactions (6.2)] including dizziness and somnolence [see Adverse Reactions (6.1)]. Patients should be monitored for signs of anticholinergic CNS effects, particularly after beginning treatment or increasing the dose. Advise patients not to drive or operate heavy machinery until the drug’s effects have been determined. If a patient experiences anticholinergic CNS effects, dose reduction or drug discontinuation should be considered.
5.6 Hepatic Impairment
The clearance of orally administered tolterodine immediate release was substantially lower in cirrhotic patients than in the healthy volunteers. For patients with mild to moderate hepatic impairment (Child-Pugh Class A or B), the recommended dose for DETROL LA is 2 mg once daily. DETROL LA is not recommended for use in patients with severe hepatic impairment (Child-Pugh Class C) [see Dosage and Administration (2.2) and Use in Specific Populations (8.6)].
5.7 Renal Impairment
Renal impairment can significantly alter the disposition of tolterodine and its metabolites. The dose of DETROL LA should be reduced to 2 mg once daily in patients with severe renal impairment (CCr: 10-30 mL/min). Patients with CCr<10 mL/min have not been studied and use of DETROL LA in this population is not recommended [see Dosage and Administration (2.2) and Use in Specific Populations (8.7)].
5.8 Myasthenia Gravis
Administer DETROL LA with caution in patients with myasthenia gravis, a disease characterized by decreased cholinergic activity at the neuromuscular junction.
5.9 Use in Patients with Congenital or Acquired QT Prolongation
In a study of the effect of tolterodine immediate release tablets on the QT interval [see Clinical Pharmacology (12.2)], the effect on the QT interval appeared greater for 8 mg/day (two times the therapeutic dose) compared to 4 mg/day and was more pronounced in CYP2D6 poor metabolizers (PM) than extensive metabolizers (EMs). The effect of tolterodine 8 mg/day was not as large as that observed after four days of therapeutic dosing with the active control moxifloxacin. However, the confidence intervals overlapped.
These observations should be considered in clinical decisions to prescribe DETROL LA to patients with a known history of QT prolongation or to patients who are taking Class IA (e.g., quinidine, procainamide) or Class III (e.g., amiodarone, sotalol) antiarrhythmic medications. There has been no association of Torsade de Pointes in the international post-marketing experience with DETROL or DETROL LA.
-
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.1 Clinical Trials Experience
The efficacy and safety of DETROL LA Capsules was evaluated in 1073 patients (537 assigned to DETROL LA; 536 assigned to placebo) who were treated with 2, 4, 6, or 8 mg/day for up to 15 months. These included a total of 1012 patients (505 randomized to DETROL LA 4 mg once daily and 507 randomized to placebo) enrolled in a randomized, placebo-controlled, double-blind, 12-week clinical efficacy and safety study.
Adverse events were reported in 52% (n=263) of patients receiving DETROL LA and in 49% (n=247) of patients receiving placebo. The most common adverse events reported by patients receiving DETROL LA were dry mouth, headache, constipation, and abdominal pain. Dry mouth was the most frequently reported adverse event for patients treated with DETROL LA, occurring in 23.4% of patients treated with DETROL LA and 7.7% of placebo-treated patients. Dry mouth, constipation, abnormal vision (accommodation abnormalities), urinary retention, and dry eyes are expected side effects of antimuscarinic agents. A serious adverse event was reported by 1.4% (n=7) of patients receiving DETROL LA and by 3.6% (n=18) of patients receiving placebo.
Table 1 lists the adverse events, regardless of causality, that were reported in the randomized, double-blind, placebo-controlled 12-week study at an incidence greater than placebo and in greater than or equal to 1% of patients treated with DETROL LA 4 mg once daily.
Table 1. Incidence* (%) of Adverse Events Exceeding Placebo Rate and Reported in ≥1% of Patients Treated with DETROL LA (4 mg daily) in a 12-week, Phase 3 Clinical Trial - * in nearest integer.
Body System
Adverse Event
% DETROL LA
n=505
% Placebo
n=507
Autonomic Nervous
dry mouth
23
8
General
headache
6
5
fatigue
2
1
Central/Peripheral
Nervousdizziness
2
1
Gastrointestinal
constipation
6
4
abdominal pain
4
2
dyspepsia
3
1
Vision
xerophthalmia
3
2
vision abnormal
1
0
Psychiatric
somnolence
3
2
anxiety
1
0
Respiratory
sinusitis
2
1
Urinary
dysuria
1
0
The frequency of discontinuation due to adverse events was highest during the first 4 weeks of treatment. Similar percentages of patients treated with DETROL LA or placebo discontinued treatment due to adverse events. Dry mouth was the most common adverse event leading to treatment discontinuation among patients receiving DETROL LA [n=12 (2.4%) vs. placebo n=6 (1.2%)].
6.2 Post-marketing Experience
The following events have been reported in association with tolterodine use in worldwide post-marketing experience:
General: anaphylaxis and angioedema; Cardiovascular: tachycardia, palpitations, peripheral edema; Gastrointestinal: diarrhea; Central/Peripheral Nervous: confusion, disorientation, memory impairment, hallucinations.
Reports of aggravation of symptoms of dementia (e.g., confusion, disorientation, delusion) have been reported after tolterodine therapy was initiated in patients taking cholinesterase inhibitors for the treatment of dementia.
Because these spontaneously reported events are from the worldwide post-marketing experience, the frequency of events and the role of tolterodine in their causation cannot be reliably determined.
-
7 DRUG INTERACTIONS
7.1 Potent CYP2D6 Inhibitors
Fluoxetine, a potent inhibitor of CYP2D6 activity, significantly inhibited the metabolism of tolterodine immediate release in CYP2D6 extensive metabolizers, resulting in a 4.8-fold increase in tolterodine AUC. There was a 52% decrease in Cmax and a 20% decrease in AUC of 5-hydroxymethyl tolterodine (5-HMT), the pharmacologically active metabolite of tolterodine [see Clinical Pharmacology (12.1)]. The sums of unbound serum concentrations of tolterodine and 5-HMT are only 25% higher during the interaction. No dose adjustment is required when tolterodine and fluoxetine are co-administered [see Clinical Pharmacology (12.3)].
7.2 Potent CYP3A4 Inhibitors
Ketoconazole (200 mg daily), a potent CYP3A4 inhibitor, increased the mean Cmax and AUC of tolterodine by 2- and 2.5-fold, respectively, in CYP2D6 poor metabolizers.
For patients receiving ketoconazole or other potent CYP3A4 inhibitors such as itraconazole, clarithromycin, or ritonavir, the recommended dose of DETROL LA is 2 mg once daily [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
7.3 Other Interactions
No clinically relevant interactions have been observed when tolterodine was co-administered with warfarin, with a combined oral contraceptive drug containing ethinyl estradiol and levonorgestrel, or with diuretics [see Clinical Pharmacology (12.3)].
7.4 Other Drugs Metabolized by Cytochrome P450 Isoenzymes
In vivo drug-interaction data show that tolterodine immediate release does not result in clinically relevant inhibition of CYP1A2, 2D6, 2C9, 2C19, or 3A4 as evidenced by lack of influence on the marker drugs caffeine, debrisoquine, S-warfarin, and omeprazole [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data with DETROL LA use in pregnant women to inform drug-associated risks. In animal reproduction studies, oral administration of tolterodine and its 5-HMT metabolite to pregnant mice during organogenesis did not produce adverse developmental outcomes at doses approximately 9 to 12 times the clinical exposure at a dose of 20 mg/kg/day; however, higher doses produced adverse developmental outcomes (see Data).
In the U.S. general population, the estimated background rate of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
No anomalies or malformations were observed after oral administration of tolterodine to pregnant mice during organogenesis at approximately 9-12 times the clinical exposure to the pharmacologically active components of DETROL LA (based on the AUC of tolterodine and its 5-HMT metabolite at a dose of 20 mg/kg/day). At 14-18 times the clinical exposure (doses of 30 to 40 mg/kg/day) in mice, tolterodine was embryo-lethal, caused reduced fetal weight, and increased the incidence of fetal abnormalities (cleft palate, digital abnormalities, intra‑abdominal hemorrhage, and various skeletal abnormalities, primarily reduced ossification). Pregnant rabbits administered tolterodine subcutaneously at about 0.3-2.5 times the clinical exposure (dose of 0.8 mg/kg/day) did not show any embryotoxicity or teratogenicity.
8.2 Lactation
Risk Summary
There is no information on the presence of tolterodine or its 5-HMT metabolite in human milk, the effects on the breastfed infant, or the effects on milk production. Based on limited data, tolterodine is excreted into the milk in mice in low amounts (see Data). The development and health benefits of breastfeeding should be considered along with the mother’s clinical need for DETROL LA and any potential adverse effects on the breastfed infant from DETROL LA or from the underlying maternal condition.
8.4 Pediatric Use
The effectiveness of DETROL LA has not been established in pediatric patients.
Efficacy was not established in two randomized, placebo-controlled, double-blind, 12-week studies that enrolled 710 pediatric patients (486 on DETROL LA, 224 on placebo) aged 5–10 years with urinary frequency and urge incontinence. The percentage of patients with urinary tract infections was higher in patients treated with DETROL LA (6.6%) compared to patients who received placebo (4.5%). Aggressive, abnormal, and hyperactive behavior and attention disorders occurred in 2.9% of children treated with DETROL LA compared to 0.9% of children treated with placebo.
8.5 Geriatric Use
No overall differences in safety were observed between the older and younger patients treated with tolterodine.
In multiple-dose studies in which tolterodine immediate release 4 mg (2 mg bid) was administered, serum concentrations of tolterodine and of 5-HMT were similar in healthy elderly volunteers (aged 64 through 80 years) and healthy young volunteers (aged less than 40 years). In another clinical study, elderly volunteers (aged 71 through 81 years) were given tolterodine immediate release 2 or 4 mg (1 or 2 mg bid). Mean serum concentrations of tolterodine and 5-HMT in these elderly volunteers were approximately 20% and 50% higher, respectively, than concentrations reported in young healthy volunteers. However, no overall differences were observed in safety between older and younger patients on tolterodine in the Phase 3, 12-week, controlled clinical studies; therefore, no tolterodine dosage adjustment for elderly patients is recommended.
8.6 Renal Impairment
Renal impairment can significantly alter the disposition of tolterodine immediate release and its metabolites. In a study conducted in patients with creatinine clearance between 10 and 30 mL/min, tolterodine and 5-HMT levels were approximately 2–3 fold higher in patients with renal impairment than in healthy volunteers. Exposure levels of other metabolites of tolterodine (e.g., tolterodine acid, N-dealkylated tolterodine acid, N-dealkylated tolterodine, and N-dealkylated hydroxy tolterodine) were significantly higher (10–30 fold) in renally impaired patients as compared to the healthy volunteers. The recommended dose for patients with severe renal impairment (CCr: 10-30 mL/min) is DETROL LA 2 mg daily. Patients with CCr<10 mL/min have not been studied and use of DETROL LA in this population is not recommended [see Dosage and Administration (2.2) and Warnings and Precautions (5.6)]. DETROL LA has not been studied in patients with mild to moderate renal impairment [CCr 30-80 mL/min].
8.7 Hepatic Impairment
Liver impairment can significantly alter the disposition of tolterodine immediate release. In a study of tolterodine immediate release conducted in cirrhotic patients (Child-Pugh Class A and B), the elimination half-life of tolterodine immediate release was longer in cirrhotic patients (mean, 7.8 hours) than in healthy, young, and elderly volunteers (mean, 2 to 4 hours). The clearance of orally administered tolterodine immediate release was substantially lower in cirrhotic patients (1.0 ± 1.7 L/h/kg) than in the healthy volunteers (5.7 ± 3.8 L/h/kg). The recommended dose for patients with mild to moderate hepatic impairment (Child-Pugh Class A or B) is DETROL LA 2 mg once daily. DETROL LA is not recommended for use in patients with severe hepatic impairment (Child-Pugh Class C) [see Dosage and Administration (2.2) and Warnings and Precautions (5.4)].
-
10 OVERDOSAGE
Overdosage with DETROL LA Capsules can potentially result in severe central anticholinergic effects and should be treated accordingly.
ECG monitoring is recommended in the event of overdosage. In dogs, changes in the QT interval (slight prolongation of 10% to 20%) were observed at a suprapharmacologic dose of 4.5 mg/kg, which is about 68 times higher than the recommended human dose. In clinical trials of normal volunteers and patients, QT interval prolongation was observed with tolterodine immediate release at doses up to 8 mg (4 mg bid) and higher doses were not evaluated [see Warnings and Precautions (5.9) and Clinical Pharmacology (12.2)].
A 27-month-old child who ingested 5 to 7 tolterodine immediate release 2 mg tablets was treated with a suspension of activated charcoal and was hospitalized overnight with symptoms of dry mouth. The child fully recovered.
-
11 DESCRIPTION
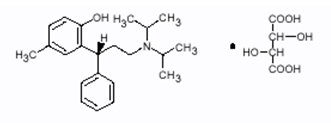
DETROL LA Capsules contain tolterodine tartrate. The active moiety, tolterodine, is a muscarinic receptor antagonist. The chemical name of tolterodine tartrate is (R)-N,N-diisopropyl-3-(2-hydroxy-5-methylphenyl)-3-phenylpropanamine L-hydrogen tartrate. The empirical formula of tolterodine tartrate is C26H37NO7. Its structure is:
Tolterodine tartrate is a white, crystalline powder with a molecular weight of 475.6. The pKa value is 9.87 and the solubility in water is 12 mg/mL. It is soluble in methanol, slightly soluble in ethanol, and practically insoluble in toluene. The partition coefficient (Log D) between n-octanol and water is 1.83 at pH 7.3.
DETROL LA 4 mg capsule for oral administration contains 4 mg of tolterodine tartrate. Inactive ingredients are sucrose, starch, hypromellose, ethylcellulose, medium chain triglycerides, oleic acid, gelatin, and FD&C Blue #2.
DETROL LA 2 mg capsule for oral administration contains 2 mg of tolterodine tartrate, and the following inactive ingredients: sucrose, starch, hypromellose, ethylcellulose, medium chain triglycerides, oleic acid, gelatin, yellow iron oxide, and FD&C Blue #2.
Both the 2 mg and 4 mg capsule strengths are imprinted with a pharmaceutical grade printing ink that contains shellac glaze, titanium dioxide, propylene glycol, and simethicone.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Tolterodine acts as a competitive antagonist of acetylcholine at postganglionic muscarinic receptors. Both urinary bladder contraction and salivation are mediated via cholinergic muscarinic receptors.
After oral administration, tolterodine is metabolized in the liver, resulting in the formation of 5-hydroxymethyl tolterodine (5-HMT), the major pharmacologically active metabolite. 5-HMT, which exhibits an antimuscarinic activity similar to that of tolterodine, contributes significantly to the therapeutic effect. Both tolterodine and 5-HMT exhibit a high specificity for muscarinic receptors, since both show negligible activity or affinity for other neurotransmitter receptors and other potential cellular targets, such as calcium channels.
12.2 Pharmacodynamics
Tolterodine has a pronounced effect on bladder function. Effects on urodynamic parameters before and 1 and 5 hours after a single 6.4 mg dose of tolterodine immediate release were determined in healthy volunteers. The main effects of tolterodine at 1 and 5 hours were an increase in residual urine, reflecting an incomplete emptying of the bladder, and a decrease in detrusor pressure. These findings are consistent with an antimuscarinic action on the lower urinary tract.
Cardiac Electrophysiology
The effect of 2 mg BID and 4 mg BID of DETROL immediate release (tolterodine IR) tablets on the QT interval was evaluated in a 4-way crossover, double-blind, placebo- and active-controlled (moxifloxacin 400 mg QD) study in healthy male (N=25) and female (N=23) volunteers aged 18–55 years. Study subjects [approximately equal representation of CYP2D6 extensive metabolizers (EMs) and poor metabolizers (PMs)] completed sequential 4-day periods of dosing with moxifloxacin 400 mg QD, tolterodine 2 mg BID, tolterodine 4 mg BID, and placebo. The 4 mg BID dose of tolterodine IR (two times the highest recommended dose) was chosen because this dose results in tolterodine exposure similar to that observed upon coadministration of tolterodine 2 mg BID with potent CYP3A4 inhibitors in patients who are CYP2D6 poor metabolizers [see Drug Interactions (7.2)]. QT interval was measured over a 12-hour period following dosing, including the time of peak plasma concentration (Tmax) of tolterodine and at steady state (Day 4 of dosing).
Table 2 summarizes the mean change from baseline to steady state in corrected QT interval (QTc) relative to placebo at the time of peak tolterodine (1 hour) and moxifloxacin (2 hour) concentrations. Both Fridericia’s (QTcF) and a population-specific (QTcP) method were used to correct QT interval for heart rate. No single QT correction method is known to be more valid than others. QT interval was measured manually and by machine, and data from both are presented. The mean increase of heart rate associated with a 4 mg/day dose of tolterodine in this study was 2.0 beats/minute and 6.3 beats/minute with 8 mg/day tolterodine. The change in heart rate with moxifloxacin was 0.5 beats/minute.
Table 2. Mean (CI) change in QTc from baseline to steady state (Day 4 of dosing) at Tmax (relative to placebo) - * At Tmax of 1 hr; 95% Confidence Interval.
- † At Tmax of 2 hr; 90% Confidence Interval.
- ‡ The effect on QT interval with 4 days of moxifloxacin dosing in this QT trial may be greater than typically observed in QT trials of other drugs.
Drug/Dose
N
QTcF
(msec)
(manual)QTcF
(msec)
(machine)
QTcP
(msec)
(manual)
QTcP
(msec)
(machine)
Tolterodine
2 mg BID*
48
5.01
(0.28, 9.74)
1.16
(-2.99, 5.30)
4.45
(-0.37, 9.26)
2.00
(-1.81, 5.81)
Tolterodine
4 mg BID*
48
11.84
(7.11, 16.58)
5.63
(1.48, 9.77)
10.31
(5.49, 15.12)
8.34
(4.53, 12.15)
Moxifloxacin
400 mg QD†45
19.26‡
(15.49, 23.03)
8.90
(4.77, 13.03)
19.10‡
(15.32, 22.89)
9.29
(5.34, 13.24)
The reason for the difference between machine and manual read of QT interval is unclear.
The QT effect of tolterodine immediate release tablets appeared greater for 8 mg/day (two times the therapeutic dose) compared to 4 mg/day. The effect of tolterodine 8 mg/day was not as large as that observed after four days of therapeutic dosing with the active control moxifloxacin. However, the confidence intervals overlapped.
Tolterodine’s effect on QT interval was found to correlate with plasma concentration of tolterodine. There appeared to be a greater QTc interval increase in CYP2D6 poor metabolizers than in CYP2D6 extensive metabolizers after tolterodine treatment in this study.
This study was not designed to make direct statistical comparisons between drugs or dose levels. There has been no association of Torsade de Pointes in the international post-marketing experience with DETROL or DETROL LA [see Warnings and Precautions (5.7)].
12.3 Pharmacokinetics
Absorption
In a study with 14C-tolterodine solution in healthy volunteers who received a 5 mg oral dose, at least 77% of the radiolabeled dose was absorbed. Cmax and area under the concentration-time curve (AUC) determined after dosage of tolterodine immediate release are dose-proportional over the range of 1 to 4 mg. Based on the sum of unbound serum concentrations of tolterodine and 5-HMT (“active moiety”), the AUC of tolterodine extended release 4 mg daily is equivalent to tolterodine immediate release 4 mg (2 mg bid). Cmax and Cmin levels of tolterodine extended release are about 75% and 150% of tolterodine immediate release, respectively. Maximum serum concentrations of tolterodine extended release are observed 2 to 6 hours after dose administration.
Distribution
Tolterodine is highly bound to plasma proteins, primarily α1-acid glycoprotein. Unbound concentrations of tolterodine average 3.7% ± 0.13% over the concentration range achieved in clinical studies. 5-HMT is not extensively protein bound, with unbound fraction concentrations averaging 36% ± 4.0%. The blood to serum ratio of tolterodine and 5-HMT averages 0.6 and 0.8, respectively, indicating that these compounds do not distribute extensively into erythrocytes. The volume of distribution of tolterodine following administration of a 1.28 mg intravenous dose is 113 ± 26.7 L.
Metabolism
Tolterodine is extensively metabolized by the liver following oral dosing. The primary metabolic route involves the oxidation of the 5-methyl group and is mediated by the cytochrome P450 2D6 (CYP2D6) and leads to the formation of a pharmacologically active metabolite, 5-HMT. Further metabolism leads to formation of the 5-carboxylic acid and N-dealkylated 5-carboxylic acid metabolites, which account for 51% ± 14% and 29% ± 6.3% of the metabolites recovered in the urine, respectively.
Variability in Metabolism
A subset of individuals (approximately 7% of Caucasians and approximately 2% of African Americans) are poor metabolizers for CYP2D6, the enzyme responsible for the formation of 5-HMT from tolterodine. The identified pathway of metabolism for these individuals (“poor metabolizers”) is dealkylation via cytochrome P450 3A4 (CYP3A4) to N-dealkylated tolterodine. The remainder of the population is referred to as “extensive metabolizers.” Pharmacokinetic studies revealed that tolterodine is metabolized at a slower rate in poor metabolizers than in extensive metabolizers; this results in significantly higher serum concentrations of tolterodine and in negligible concentrations of 5-HMT.
Excretion
Following administration of a 5 mg oral dose of 14C-tolterodine solution to healthy volunteers, 77% of radioactivity was recovered in urine and 17% was recovered in feces in 7 days. Less than 1% (< 2.5% in poor metabolizers) of the dose was recovered as intact tolterodine, and 5% to 14% (<1% in poor metabolizers) was recovered as 5-HMT.
A summary of mean (± standard deviation) pharmacokinetic parameters of tolterodine extended release and 5-HMT in extensive (EM) and poor (PM) metabolizers is provided in Table 3. These data were obtained following single and multiple doses of tolterodine extended release administered daily to 17 healthy male volunteers (13 EM, 4 PM).
Table 3. Summary of Mean (±SD) Pharmacokinetic Parameters of Tolterodine Extended Release and its Active Metabolite (5-Hydroxymethyl Tolterodine) in Healthy Volunteers Cmax = Maximum serum concentration; tmax = Time of occurrence of Cmax; Cavg = Average serum concentration; t1/2 = Terminal elimination half-life. - * Data presented as median (range).
- † Parameter dose-normalized from 8 to 4 mg for the single-dose data.
- ‡ = not applicable.
Tolterodine
5-Hydroxymethyl Tolterodine
tmax*
(h)
Cmax
(µg/L)Cavg
(µg/L)
t½
(h)
tmax*
(h)
Cmax
(µg/L)
Cavg
(µg/L)
t½
(h)
Single dose
4 mg†
EM
4 (2–6)
1.3 (0.8)
0.8 (0.57)
8.4 (3.2)
4 (3–6)
1.6 (0.5)
1.0 (0.32)
8.8 (5.9)
Multiple
dose 4 mg
EM
PM
4 (2–6)
4 (3–6)
3.4 (4.9)
19 (16)
1.7 (2.8)
13 (11)
6.9 (3.5)
18 (16)
4 (2–6)
2.7 (0.90)
1.4 (0.6)
9.9 (4.0)
Drug Interactions
Potent CYP2D6 Inhibitors
Fluoxetine is a selective serotonin reuptake inhibitor and a potent inhibitor of CYP2D6 activity. In a study to assess the effect of fluoxetine on the pharmacokinetics of tolterodine immediate release and its metabolites, it was observed that fluoxetine significantly inhibited the metabolism of tolterodine immediate release in extensive metabolizers, resulting in a 4.8-fold increase in tolterodine AUC. There was a 52% decrease in Cmax and a 20% decrease in AUC of 5-hydroxymethyl tolterodine (5-HMT, the pharmacologically active metabolite of tolterodine). Fluoxetine thus alters the pharmacokinetics in patients who would otherwise be CYP2D6 extensive metabolizers of tolterodine immediate release to resemble the pharmacokinetic profile in poor metabolizers. The sums of unbound serum concentrations of tolterodine immediate release and 5-HMT are only 25% higher during the interaction. No dose adjustment is required when tolterodine and fluoxetine are co-administered.
Potent CYP3A4 Inhibitors
The effect of a 200 mg daily dose of ketoconazole on the pharmacokinetics of tolterodine immediate release was studied in 8 healthy volunteers, all of whom were CYP2D6 poor metabolizers. In the presence of ketoconazole, the mean Cmax and AUC of tolterodine increased by 2- and 2.5-fold, respectively. Based on these findings, other potent CYP3A4 inhibitors may also lead to increases of tolterodine plasma concentrations.
For patients receiving ketoconazole or other potent CYP3A4 inhibitors such as itraconazole, miconazole, clarithromycin, ritonavir, the recommended dose of DETROL LA is 2 mg daily [see Dosage and Administration (2.3)].
Warfarin
In healthy volunteers, coadministration of tolterodine immediate release 4 mg (2 mg bid) for 7 days and a single dose of warfarin 25 mg on day 4 had no effect on prothrombin time, Factor VII suppression, or on the pharmacokinetics of warfarin.
Oral Contraceptives
Tolterodine immediate release 4 mg (2 mg bid) had no effect on the pharmacokinetics of an oral contraceptive (ethinyl estradiol 30 µg/levonorgestrel 150 µg) as evidenced by the monitoring of ethinyl estradiol and levonorgestrel over a 2-month period in healthy female volunteers.
Diuretics
Coadministration of tolterodine immediate release up to 8 mg (4 mg bid) for up to 12 weeks with diuretic agents, such as indapamide, hydrochlorothiazide, triamterene, bendroflumethiazide, chlorothiazide, methylchlorothiazide, or furosemide, did not cause any adverse electrocardiographic (ECG) effects.
Effect of Tolterodine on Other Drugs Metabolized by Cytochrome P450 Enzymes
Tolterodine immediate release does not cause clinically significant interactions with other drugs metabolized by the major drug-metabolizing CYP enzymes. In vivo drug-interaction data show that tolterodine immediate release does not result in clinically relevant inhibition of CYP1A2, 2D6, 2C9, 2C19, or 3A4 as evidenced by lack of influence on the marker drugs caffeine, debrisoquine, S-warfarin, and omeprazole. In vitro data show that tolterodine immediate release is a competitive inhibitor of CYP2D6 at high concentrations (Ki 1.05 µM), while tolterodine immediate release as well as the 5-HMT are devoid of any significant inhibitory potential regarding the other isoenzymes.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies with tolterodine were conducted in mice and rats. At the maximum tolerated dose in mice (30 mg/kg/day), female rats (20 mg/kg/day), and male rats (30 mg/kg/day), exposure margins were approximately 6-9 times, 7 times, and 11 times the clinical exposure to the pharmacologically active components of DETROL LA (based on AUC of tolterodine and its 5-HMT metabolite). At these exposure margins, no increase in tumors was found in either mice or rats.
No mutagenic or genotoxic effects of tolterodine were detected in a battery of in vitro tests, including bacterial mutation assays (Ames test) in 4 strains of Salmonella typhimurium and in 2 strains of Escherichia coli, a gene mutation assay in L5178Y mouse lymphoma cells, and chromosomal aberration tests in human lymphocytes. Tolterodine was also negative in vivo in the bone marrow micronucleus test in the mouse.
In female mice treated for 2 weeks before mating and during gestation with 20 mg/kg/day (about 9-12 times the clinical exposure via AUC), neither effects on reproductive performance or fertility were seen. In male mice, a dose of 30 mg/kg/day did not induce any adverse effects on fertility.
-
14 CLINICAL STUDIES
DETROL LA Capsules 2 mg were evaluated in 29 patients in a Phase 2 dose-effect study. DETROL LA 4 mg was evaluated for the treatment of overactive bladder with symptoms of urge urinary incontinence and frequency in a randomized, placebo-controlled, multicenter, double-blind, Phase 3, 12-week study. A total of 507 patients received DETROL LA 4 mg once daily in the morning and 508 received placebo. The majority of patients were Caucasian (95%) and female (81%), with a mean age of 61 years (range, 20 to 93 years). In the study, 642 patients (42%) were 65 to 93 years of age. The study included patients known to be responsive to tolterodine immediate release and other anticholinergic medications, however, 47% of patients never received prior pharmacotherapy for overactive bladder. At study entry, 97% of patients had at least 5 urge incontinence episodes per week and 91% of patients had 8 or more micturitions per day.
The primary efficacy assessment was change in mean number of incontinence episodes per week at week 12 from baseline. Secondary efficacy measures included change in mean number of micturitions per day and mean volume voided per micturition at week 12 from baseline.
Patients treated with DETROL LA experienced a statistically significant decrease in number of urinary incontinence per week from baseline to last assessment (week 12) compared with placebo as well as a decrease in the average daily urinary frequency and an increase in the average urine volume per void.
Mean change from baseline in weekly incontinence episodes, urinary frequency, and volume voided between placebo and DETROL LA are summarized in Table 4.
Table 4. 95% Confidence Intervals (CI) for the Difference between DETROL LA (4 mg daily) and Placebo for Mean Change at Week 12 from Baseline* SD = Standard Deviation. - * Intent-to-treat analysis.
- † 1 to 2 patients missing in placebo group for each efficacy parameter.
- ‡ The difference between DETROL LA and placebo was statistically significant.
DETROL LA
(n=507)
Placebo
(n=508)†
Treatment
Difference, vs.
Placebo(95% Cl)
Number of incontinence
episodes/weekMean Baseline
Mean Change from Baseline
22.1
–11.8 (SD 17.8)
23.3
–6.9 (SD 15.4)
-4.8‡
(–6.9, –2.8)
Number of micturitions/day
Mean Baseline
Mean Change from Baseline
10.9
–1.8 (SD 3.4)
11.3
–1.2 (SD 2.9)
-0.6‡
(–1.0, –0.2)
Volume voided per micturition
(mL)Mean Baseline
Mean Change from Baseline
141
34 (SD 51)
136
14 (SD 41)
20‡
(14, 26)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
DETROL LA Capsules are supplied as follows:
Bottles of 30
Bottles of 500
2 mg Capsules
NDC: 58151-103-93
2 mg Capsules
NDC: 58151-103-05
4 mg Capsules
NDC: 58151-104-93
4 mg Capsules
NDC: 58151-104-05
Bottles of 90
2 mg Capsules
NDC: 58151-103-77
4 mg Capsules
NDC: 58151-104-77
Store at 20°–25°C (68°–77°F); excursions permitted to 15–30°C (59–86°F) [see USP Controlled Room Temperature]. Protect from light.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Antimuscarinic Effects
Inform patients that antimuscarinic agents such as DETROL LA may have side effects including blurred vision, dizziness, or drowsiness. Advise patients not to drive, operate machinery, or do other potentially dangerous activities until they know how DETROL LA affects them.
Distributed by:
Viatris Specialty LLC
Morgantown, WV 26505 U.S.A.© 2023 Viatris Inc.
DETROL is a registered trademark of Viatris Specialty LLC, a Viatris Company.
UPJ:DTRLLAC:R1
-
PATIENT INFORMATION
DETROL® LA (DE-trol el-ay)
(tolterodine tartrate extended release capsules)Read the Patient Information that comes with DETROL LA before you start using it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your condition or your treatment. Only your doctor can determine if treatment with DETROL LA is right for you.
What is DETROL LA?
DETROL LA is a prescription medicine for adults used to treat the following symptoms due to a condition called overactive bladder:
- Having a strong need to urinate with leaking or wetting accidents (urge urinary incontinence).
- Having a strong need to urinate right away (urgency).
- Having to urinate often (frequency).
DETROL LA did not help the symptoms of overactive bladder when studied in children.
What is overactive bladder?
Overactive bladder happens when you cannot control your bladder muscle. When the muscle contracts too often or cannot be controlled, you get symptoms of overactive bladder, which are leakage of urine (urge urinary incontinence), needing to urinate right away (urgency), and needing to urinate often (frequency).
Who should not take DETROL LA?
Do not take DETROL LA if:
- You have trouble emptying your bladder (also called “urinary retention”).
- Your stomach empties slowly (also called “gastric retention”).
- You have an eye problem called “uncontrolled narrow-angle glaucoma”.
- You are allergic to DETROL LA or to any of its ingredients. See the end of this leaflet for a complete list of ingredients.
- You are allergic to TOVIAZ®, which contains fesoterodine.
What should I tell my doctor before starting DETROL LA?
Before starting DETROL LA, tell your doctor about all of your medical conditions, including if you:
- Have any stomach or intestinal problems.
- Have trouble emptying your bladder or you have a weak urine stream.
- Have an eye problem called narrow-angle glaucoma.
- Have liver problems.
- Have kidney problems.
- Have a condition called myasthenia gravis.
- Or any family members have a rare heart condition called QT prolongation (long QT syndrome).
- Are pregnant or trying to become pregnant. It is not known if DETROL LA could harm your unborn baby.
- Are breastfeeding. It is not known if DETROL LA passes into your milk and if it can harm your child.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Other drugs can affect how your body handles DETROL LA. Your doctor may use a lower dose of DETROL LA if you are taking:
- Certain medicines for fungus or yeast infections such as Nizoral® (ketoconazole), Sporanox® (itraconazole), or Monistat® (miconazole).
- Certain medicines for bacteria infections such as Biaxin® (clarithromycin).
- Certain medicines for treatment of HIV infection such as Norvir® (ritonavir), Invirase® (saquinavir), Reyataz® (atazanavir).
- Sandimmune® (cyclosporine) or Velban® (vinblastine).
Know the medicines you take. Keep a list of them with you to show your doctor or pharmacist each time you get a new medicine.
How should I take DETROL LA?
- Take DETROL LA exactly as prescribed. Your doctor will prescribe the dose that is right for you. Do not change your dose unless told to do so by your doctor.
- Take DETROL LA capsules once a day with liquid. Swallow the whole capsule. Tell your doctor if you cannot swallow a capsule.
- DETROL LA can be taken with or without food.
- Take DETROL LA the same time each day.
- If you miss a dose of DETROL LA, begin taking DETROL LA again the next day. Do not take 2 doses of DETROL LA in the same day.
- If you took more than your prescribed dose of DETROL LA, call your doctor or poison control center, or go to the hospital emergency room.
What are possible side effects of DETROL LA?
DETROL LA may cause allergic reactions that may be serious. Symptoms of a serious allergic reaction may include swelling of the face, lips, throat, or tongue. If you experience these symptoms, you should stop taking DETROL LA and get emergency medical help right away.
The most common side effects with DETROL LA are:
- Dry mouth
- Constipation
- Headache
- Stomach pain
Medicines like DETROL LA can cause blurred vision, dizziness, and drowsiness.
Do not drive, operate machinery, or do other dangerous activities until you know how DETROL LA affects you.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
These are not all the side effects with DETROL LA. For a complete list, ask your doctor or pharmacist.
How do I store DETROL LA?
- Store DETROL LA at room temperature, 68° - 77°F (20° - 25°C); brief periods permitted between 59° - 86°F (15° - 30°C). Protect from light. Keep in a dry place.
- Keep DETROL LA and all medicines out of the reach of children.
General Information about DETROL LA
Medicines are sometimes prescribed for conditions that are not in the patient information leaflet. Only use DETROL LA the way your doctor tells you. Do not share it with other people even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about DETROL LA. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about DETROL LA that is written for health professionals. You can also call Viatris at 1-877-446-3679 (1-877-4-INFO-RX).
What are the ingredients in DETROL LA?
Active ingredients: tolterodine tartrate
Inactive ingredients: sucrose, starch, hypromellose, ethylcellulose, medium chain triglycerides, oleic acid, gelatin, and FD&C Blue #2. 2 mg capsule also contains yellow iron oxide. Capsules have pharmaceutical grade printing ink that contains shellac glaze, titanium dioxide, propylene glycol, and simethicone.
Distributed by:
Viatris Specialty LLC
Morgantown, WV 26505 U.S.A.© 2023 Viatris Inc.
DETROL is a registered trademark of Viatris Specialty LLC, a Viatris Company.
The brands listed are trademarks of their respective owners.
UPJ:PL:DTRLLAC:R1
Revised: 3/2023
-
PRINCIPAL DISPLAY PANEL – 2 mg
NDC: 58151-103-93
Detrol® LA
tolterodine tartrate
extended release capsules2 mg
30 Capsules Rx only
VIATRIS™
Store at 25ºC (77ºF);
excursions permitted to
15-30ºC (59-86ºF)
[see USP Controlled
Room Temperature].Protect from light.
DOSAGE AND USE
See package insert for full
prescribing information.Each capsule contains
2 mg tolterodine tartrate.Distributed by:
Viatris Specialty LLC
Morgantown, WV 26505
U.S.A.© 2023 Viatris Inc.
RUPJ103H
-
PRINCIPAL DISPLAY PANEL – 4 mg
NDC: 58151-104-93
Detrol® LA
tolterodine tartrate
extended release capsules4 mg
30 Capsules Rx only
VIATRIS™
Store at 25ºC (77ºF);
excursions permitted to
15-30ºC (59-86ºF)
[see USP Controlled
Room Temperature].Protect from light.
DOSAGE AND USE
See package insert for full
prescribing information.Each capsule contains
4 mg tolterodine tartrate.Distributed by:
Viatris Specialty LLC
Morgantown, WV 26505
U.S.A.© 2023 Viatris Inc.
RUPJ104H
-
INGREDIENTS AND APPEARANCE
DETROL LA
tolterodine tartrate capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 58151-103 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOLTERODINE TARTRATE (UNII: 5T619TQR3R) (TOLTERODINE - UNII:WHE7A56U7K) TOLTERODINE TARTRATE 2 mg Inactive Ingredients Ingredient Name Strength ETHYLCELLULOSE, UNSPECIFIED (UNII: 7Z8S9VYZ4B) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) OLEIC ACID (UNII: 2UMI9U37CP) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) SUCROSE (UNII: C151H8M554) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color BLUE (blue-green) Score no score Shape CAPSULE Size 14mm Flavor Imprint Code 2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58151-103-93 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/10/2024 07/31/2026 2 NDC: 58151-103-77 90 in 1 BOTTLE; Type 0: Not a Combination Product 01/10/2024 07/31/2026 3 NDC: 58151-103-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 07/31/2026 07/31/2026 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021228 01/10/2024 07/31/2026 DETROL LA
tolterodine tartrate capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 58151-104 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOLTERODINE TARTRATE (UNII: 5T619TQR3R) (TOLTERODINE - UNII:WHE7A56U7K) TOLTERODINE TARTRATE 4 mg Inactive Ingredients Ingredient Name Strength ETHYLCELLULOSE, UNSPECIFIED (UNII: 7Z8S9VYZ4B) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) OLEIC ACID (UNII: 2UMI9U37CP) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) SUCROSE (UNII: C151H8M554) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color BLUE Score no score Shape CAPSULE Size 16mm Flavor Imprint Code 4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58151-104-93 30 in 1 BOTTLE; Type 0: Not a Combination Product 03/20/2024 07/31/2026 2 NDC: 58151-104-77 90 in 1 BOTTLE; Type 0: Not a Combination Product 03/20/2024 07/31/2026 3 NDC: 58151-104-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 10/31/2025 10/31/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021228 03/20/2024 07/31/2026 Labeler - Viatris Specialty LLC (117455616)
Trademark Results [Detrol LA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DETROL LA 78310337 not registered Dead/Abandoned |
PHARMACIA ENTERPRISES S.A. 2003-10-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.