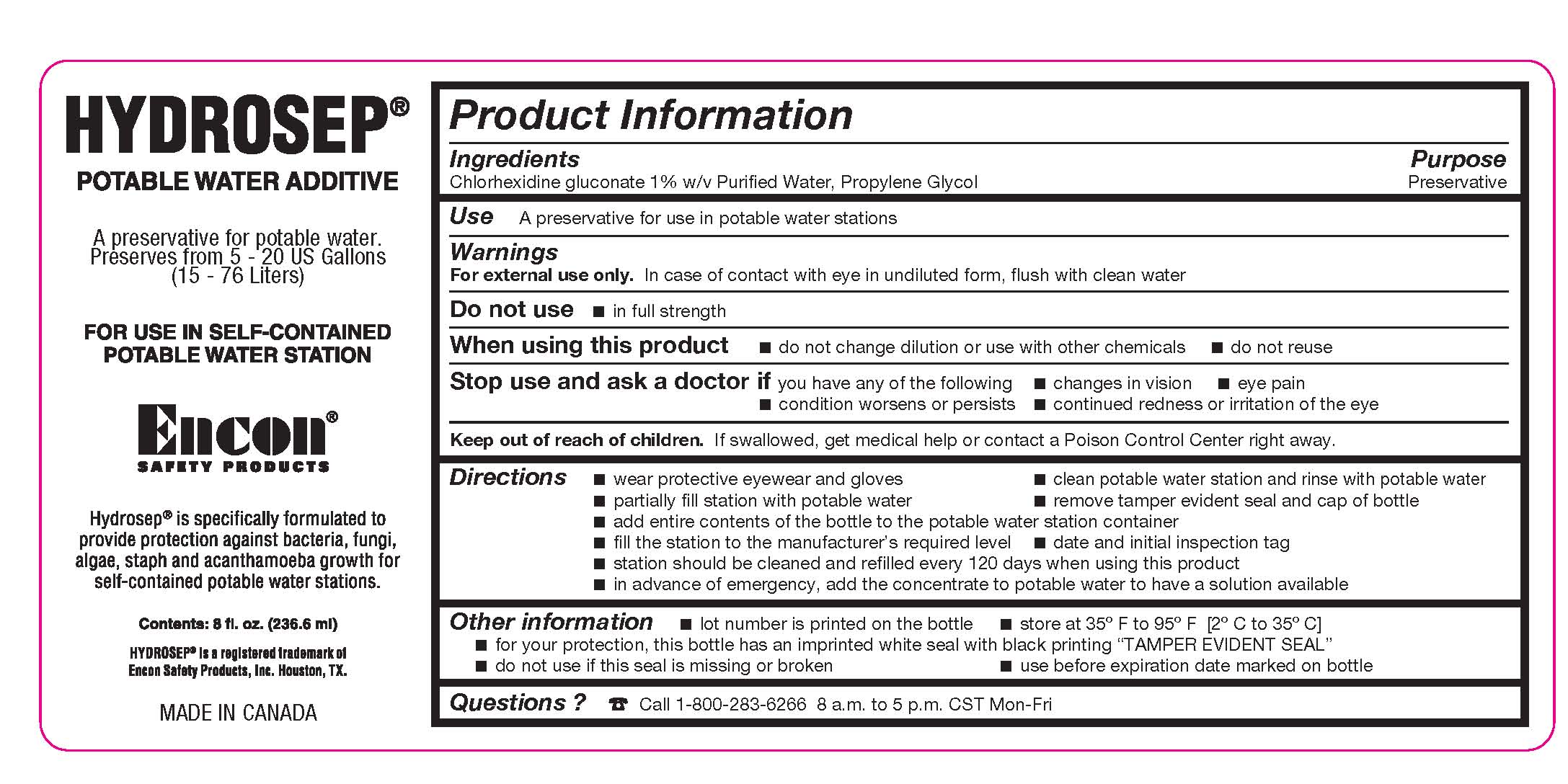
Eyewash Station Additive Concentrate by Niagara Pharmaceuticals Inc. Encon Additive
Eyewash Station Additive Concentrate by
Drug Labeling and Warnings
Eyewash Station Additive Concentrate by is a Otc medication manufactured, distributed, or labeled by Niagara Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
EYEWASH STATION ADDITIVE CONCENTRATE- chlorhexidine gluconate and propylene glycol liquid
Niagara Pharmaceuticals Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Encon Additive
Warnings
For external use only. In case of contact with eye in undiluted form, flush with clean water
Directions
- wear protective eyewear and gloves
- clean potable eyewash station and rinse with potable water
- partially fill station with potable water
- remove tamper evident seal and cap of bottle
- add entire contents of the bottle to the eyewash station container
- fill the station to the manufacturer's required level
- date and initial inspection tag
- station should be cleaned and refilled every 120 days when using this product
- in advance of emergency, add the concentrate to potable water to have a solution available
| EYEWASH STATION ADDITIVE CONCENTRATE
chlorhexidine gluconate and propylene glycol liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Niagara Pharmaceuticals Inc. (205477792) |
| Registrant - Niagara Pharmaceuticals Inc. (205477792) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Niagara Pharmaceuticals Inc. | 205477792 | manufacture(65785-038) | |
Revised: 2/2022
Document Id: d736fbd6-4cfd-bc33-e053-2a95a90a26fd
Set id: d736fbd6-4cfc-bc33-e053-2a95a90a26fd
Version: 1
Effective Time: 20220204