TOLAZINE- tolazoline hydrochloride injection, solution
Tolazine by
Drug Labeling and Warnings
Tolazine by is a Animal medication manufactured, distributed, or labeled by Akorn Animal Health, Inc., Akorn Operating Company LLC, Akorn, Inc, AMRI Rensselaer, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION:
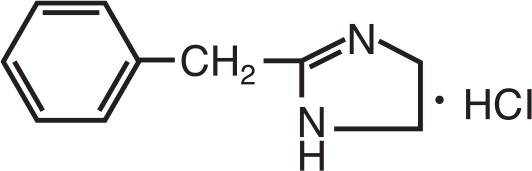
Tolazine contains tolazoline hydrochloride with the chemical name 1H-Imidazole,4,5-dihydro-2-(phenylmethyl)-monohydrochloride. Tolazoline hydrochloride has a molecular weight of 196.68 and the molecular formula is C10H12N2HCl. The structural formula is
Each mL contains: tolazoline hydrochloride equivalent to 100 mg base activity, chlorobutanol 5.0 mg, tartaric acid 7.8 mg, sodium citrate dihydrate 7.8 mg and water for injection. The pH is adjusted with hydrochloric acid and sodium citrate.
- INDICATIONS:
-
DOSAGE AND ADMINISTRATION:
The Tolazine dose is 4.0 mg/kg body weight or 1.8 mg/lb (4 mL/100 kg or 4 mL/220 lb) to reverse the sedative effects of xylazine. The carefully calculated dose of Tolazine should be given slowly by intravenous injection. Administration rate should approximate 1 mL/second.1,5
The table demonstrates the correct injection volume based on body weight:
Body Weight (kg)
Body Weight (lb)
Tolazine Injection Volume250 kg 550 lb 10 mL 500 kg 1100 lb 20 mL -
WARNING:
Keep out of reach of children. Not for human use.
Do not use in horses intended for human consumption.Avoid contact with eyes, skin and mucous membranes. In case of eye contact flush with plenty of water. Exposed skin should be washed with soap and water. In case of accidental oral exposure or injection, seek emergency medical attention.
Users with cardiovascular disease (for example, hypertension or ischemic heart disease) should take special precautions to avoid accidental exposure to this product.
To report adverse reactions in users or to obtain a copy of the material safety data sheet for this product, call 1-800-932-5676.
-
PRECAUTIONS:
The safety of Tolazine has not been evaluated in horses with metabolically unstable conditions. Tolazine should not be administered to animals exhibiting signs of stress, debilitation, cardiac disease, sympathetic blockage, hypovolemia or shock.
The safety of Tolazine has not been evaluated for reversing xylazine used as a preanesthetic to a general anesthetic.
The safety of Tolazine has not been established in pregnant mares, lactating mares, horses intended for breeding, or foals.
Tolazine should be administered carefully and at a slow rate to allow venous dilution to occur prior to the drug reaching the brain and heart. An administration rate of 1 mL/second was shown to be safe at the recommended dose.
Tolazine reverses the analgesic effects of xylazine as well as the sedative effects. If the animal was given xylazine for its analgesic properties, reversal may result in return of normal pain perception.
-
ADVERSE REACTIONS:
Temporary side effects of Tolazine may be mild increases in blood pressure; tachycardia; peripheral vasodilatation, evidenced by injected mucous membranes of the gingiva and conjunctiva; and sweating. A few horses may show hyperalgesia of the lips, evidenced by licking or flipping of the lips. Some horses may exhibit piloerection soon after dosing. Clear lacrimal and nasal discharges may be noted. Muscle fasciculations may be observed. Some horses may show signs of temporary apprehensiveness. All clinical side effects should dissipate within 60 to 120 minutes.
The potential for side effects increases when higher than recommended doses are given or when Tolazine is given without prior administration of xylazine.
NOTE TO PHYSICIAN: This product contains an alpha-2 adrenoreceptor antagonist. Epinephrine should not be used to treat hypotension in humans resulting from exposure to this product since tolazoline may cause “epinephrine reversal” (further reduction in blood pressure, followed by an exaggerated rebound).
-
CLINICAL PHARMACOLOGY:
Tolazoline belongs to the synthetic group of alpha-adrenergic blocking agents known as the imidazoline derivatives.1 It is a mixed alpha-1 and alpha-2 adrenergic receptor antagonist that competitively inhibits alpha-adrenoceptors.2,3 Tolazoline is also a direct peripheral vasodilator that decreases the peripheral resistance and increases venous capacitance.3
Xylazine is an alpha-2 adrenergic agonist with sedative and analgesic properties related to central nervous system depression. Administration of Tolazine reverses xylazine's central nervous system depressant effects resulting in rapid recovery from sedation. The competitive blocking of the alpha-2 adrenergic receptor by Tolazine displaces xylazine from these sites and thereby rapidly cancels the effect of the xylazine.4
Onset of arousal is usually apparent within 5 minutes of Tolazine administration, depending on the depth and duration of xylazine induced sedation.4
-
SAFETY:
The safety of Tolazine alone without prior xylazine administration was evaluated in healthy horses. Tolazine was administered at 1, 3, and 5 times the recommended dose of 4 mg/kg, every 6 hours for 3 doses. When administered alone, Tolazine caused gastrointestinal hypermotility as horses defecated or attempted to defecate with flatulence within minutes after injection. Some horses exhibited abdominal discomfort (mild colic) and displayed transient diarrhea. Gastrointestinal disturbances were seen in all dose groups.
The safety of Tolazine was evaluated following administration of xylazine in healthy horses. The frequency of gastrointestinal disturbances was decreased in the presence of xylazine. Most horses experienced xylazine-induced hypomotility. A return to normal intestinal motility occurred within 5 minutes after Tolazine administration. A single incidence of mild colic was observed at three times the recommended Tolazine dose, and one instance of transient diarrhea was exhibited at three times the recommended Tolazine dose.
The heart rate may briefly increase immediately after Tolazine injection at the recommended dose with return to pretreatment rates within 5 to 10 minutes. The degree and duration of tachycardia increased with higher doses, although the increased rate usually lasted less than 60 minutes.
When Tolazine was administered at higher than the recommended dose in healthy horses, intraventricular conduction was slowed, as demonstrated by a prolongation of the QRS complex of the ECG. This effect was not observed at the 1X dose, only a mild prolongation occurred in a single horse at the 2X dose, and it was seen in most horses that received the 3X and 5X doses. Abnormalities of intraventricular conduction can predispose to ventricular arrhythmias and possibly death.
Overdoses of Tolazine at 5 times the recommended dose have been associated with fatalities in horses.
Tolazine reverses blood pressure reduction induced by xylazine in healthy horses, and may result in mild hypertension in some animals at the recommended dose.
Tolazine at doses less than or equal to 5 times the recommended dose did not affect any hematologic, serum biochemical, or urinalysis measurements.
- STORAGE:
- HOW SUPPLIED:
-
REFERENCES:
- Adams, H.R. Veterinary Pharmacology and Therapeutics. Iowa State University Press, Ames, IA. 7th ed. 1995:104-108.
- Short, C.E. Ed.: Principles and Practice of Veterinary Anesthesia. Williams and Wilkins, Baltimore, MD, 1987.
- Tranquilli, W.J. and Thurmon, J.C. Alpha adrenoceptor pharmacology. JAVMA 1984; 184:1400-1402.
- LLOYD, Inc. Research, 1989-1995.
- Hsu, W.H., et al. Effects of tolazoline and yohimbine on xylazine-induced central nervous system depression, bradycardia, and tachypnea in sheep. JAVMA 1987; 190:423-426.
AKORN ANIMAL HEALTH
Manufactured by:
Akorn, Inc.
Lake Forest, IL 60045TH00N Rev. 05/18
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Container Label:
NDC: 59399-113-90
Tolazine®
INJECTION
(tolazoline hydrochloride injection)
100 mg Tolazoline per mLXylazine Reversing Agent and Antagonist
for Intravenous Use in Horses Only
100 mL
CAUTION: Federal law restricts this drug to use
by or on the order of a licensed veterinarian.
NADA #140-994, Approved by FDA
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Carton Label:
NDC: 59399-113-90
Tolazine®
INJECTION
(tolazoline hydrochloride injection)
100 mg Tolazoline per mL
Xylazine Reversing Agent
and Antagonist for Intravenous
Use in Horses Only
100 mL
CAUTION: Federal law restricts
this drug to use by or on the
order of a licensed veterinarian.
Akorn Animal Health Logo
NADA #140-994, Approved by FDA
-
INGREDIENTS AND APPEARANCE
TOLAZINE
tolazoline hydrochloride injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 59399-113 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Tolazoline Hydrochloride (UNII: E669Z6S1JG) (Tolazoline - UNII:CHH9H12AQ3) Tolazoline 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength Chlorobutanol (UNII: HM4YQM8WRC) 5.0 mg in 1 mL Tartaric Acid (UNII: W4888I119H) 7.8 mg in 1 mL Trisodium Citrate Dihydrate (UNII: B22547B95K) 708 mg in 1 mL Water (UNII: 059QF0KO0R) Hydrochloric Acid (UNII: QTT17582CB) Sodium Citrate (UNII: 1Q73Q2JULR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59399-113-90 1 in 1 CARTON 1 100 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA140994 02/26/2015 Labeler - Akorn Animal Health, Inc. (078876357) Establishment Name Address ID/FEI Business Operations Akorn, Inc 603980319 MANUFACTURE, ANALYSIS, STERILIZE, PACK, LABEL Establishment Name Address ID/FEI Business Operations AMRI Rensselaer, Inc. 124193793 API MANUFACTURE
Trademark Results [Tolazine]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TOLAZINE 76548343 2905937 Live/Registered |
Akorn Animal Health, Inc. 2003-10-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.