RELENZA- zanamivir powder
RELENZA by
Drug Labeling and Warnings
RELENZA by is a Prescription medication manufactured, distributed, or labeled by GlaxoSmithKline LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use RELENZA safely and effectively. See full prescribing information for RELENZA.
RELENZA (zanamivir inhalation powder), for oral inhalation use
Initial U.S. Approval: 1999INDICATIONS AND USAGE
RELENZA, an influenza virus neuraminidase inhibitor (NAI), is indicated for:
Treatment of acute, uncomplicated influenza type A and B infections in patients aged 7 years and older who have been symptomatic for no more than 2 days. (1.1)
Prophylaxis of influenza in patients aged 5 years and older. (1.2)
Important Limitations of Use:
Not recommended for treatment or prophylaxis of influenza in:
- Individuals with underlying airways disease. (5.1)
Not proven effective for:
- Treatment in individuals with underlying airways disease. (1.3)
- Prophylaxis in nursing home residents. (1.3)
Not a substitute for annual influenza vaccination. (1.3)
Consider available information on influenza drug susceptibility patterns and treatment effects when deciding whether to use RELENZA. (1.3)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Blister for oral inhalation: 5 mg. Four 5-mg blisters of powder on a ROTADISK for oral inhalation via DISKHALER. Packaged in carton containing 5 ROTADISKs (total of 10 doses) and 1 DISKHALER inhalation device. (3)
CONTRAINDICATIONS
Do not use in patients with history of allergic reaction to any ingredient of RELENZA, including milk proteins. (4)
WARNINGS AND PRECAUTIONS
- Bronchospasm: Serious, sometimes fatal, cases have occurred. Not recommended in individuals with underlying airways disease. Discontinue RELENZA if bronchospasm or decline in respiratory function develops. (5.1)
- Allergic Reactions: Discontinue RELENZA and initiate appropriate treatment if an allergic reaction occurs or is suspected. (5.2)
- Neuropsychiatric Events: Patients with influenza, particularly pediatric patients, may be at an increased risk of seizures, confusion, or abnormal behavior early in their illness. Monitor for signs of abnormal behavior. (5.3)
- High-Risk Underlying Medical Conditions: Safety and effectiveness have not been demonstrated in these patients. (5.4)
ADVERSE REACTIONS
The most common adverse events reported in greater than 1.5% of subjects treated with RELENZA and more commonly than in subjects treated with placebo are:
- Treatment Trials – sinusitis, dizziness. (6.1)
- Prophylaxis Trials – fever and/or chills, arthralgia, and articular rheumatism. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact GlaxoSmithKline at 1-888-825-5249 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Live attenuated influenza vaccine, intranasal (7):
- Do not administer until 48 hours following cessation of RELENZA.
- Do not administer RELENZA until 2 weeks following administration of the live attenuated influenza vaccine, unless medically indicated.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Treatment of Influenza
1.2 Prophylaxis of Influenza
1.3 Important Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Considerations
2.2 Treatment of Influenza
2.3 Prophylaxis of Influenza
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Bronchospasm
5.2 Allergic Reactions
5.3 Neuropsychiatric Events
5.4 Limitations of Populations Studied
5.5 Bacterial Infections
5.6 Importance of Proper Route of Administration
5.7 Importance of Proper Use of DISKHALER
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Treatment of Influenza
14.2 Prophylaxis of Influenza
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Treatment of Influenza
RELENZA (zanamivir) inhalation powder is indicated for treatment of uncomplicated acute illness due to influenza A and B virus in adults and pediatric patients aged 7 years and older who have been symptomatic for no more than 2 days.
1.2 Prophylaxis of Influenza
RELENZA is indicated for prophylaxis of influenza in adults and pediatric patients aged 5 years and older.
1.3 Important Limitations of Use
- RELENZA is not recommended for treatment or prophylaxis of influenza in individuals with underlying airways disease (such as asthma or chronic obstructive pulmonary disease) due to risk of serious bronchospasm [see Warnings and Precautions (5.1)].
- RELENZA has not been proven effective for treatment of influenza in individuals with underlying airways disease.
- RELENZA has not been proven effective for prophylaxis of influenza in the nursing home setting.
- RELENZA is not a substitute for early influenza vaccination on an annual basis as recommended by the Centers for Disease Control's Immunization Practices Advisory Committee.
- Influenza viruses change over time. Emergence of resistance mutations could decrease drug effectiveness. Other factors (for example, changes in viral virulence) might also diminish clinical benefit of antiviral drugs. Prescribers should consider available information on influenza drug susceptibility patterns and treatment effects when deciding whether to use RELENZA.
- There is no evidence for efficacy of zanamivir in any illness caused by agents other than influenza virus A and B.
- Patients should be advised that the use of RELENZA for treatment of influenza has not been shown to reduce the risk of transmission of influenza to others.
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Considerations
- RELENZA is for administration to the respiratory tract by oral inhalation only, using the DISKHALER device provided [see Warnings and Precautions (5.6)].
- The 10-mg dose is provided by 2 inhalations (one 5-mg blister per inhalation).
- Patients should be instructed in the use of the delivery system. Instructions should include a demonstration whenever possible. If RELENZA is prescribed for children, it should be used only under adult supervision and instruction, and the supervising adult should first be instructed by a healthcare professional [see Patient Counseling Information (17)].
- Patients scheduled to use an inhaled bronchodilator at the same time as RELENZA should use their bronchodilator before taking RELENZA [see Patient Counseling Information (17)].
2.2 Treatment of Influenza
- The recommended dose of RELENZA for treatment of influenza in adults and pediatric patients aged 7 years and older is 10 mg twice daily (approximately 12 hours apart) for 5 days.
- Two doses should be taken on the first day of treatment whenever possible provided there is at least 2 hours between doses.
- On subsequent days, doses should be about 12 hours apart (e.g., morning and evening) at approximately the same time each day.
- The safety and efficacy of repeated treatment courses have not been studied.
2.3 Prophylaxis of Influenza
Household Setting
- The recommended dose of RELENZA for prophylaxis of influenza in adults and pediatric patients aged 5 years and older in a household setting is 10 mg once daily for 10 days.
- The dose should be administered at approximately the same time each day.
- There are no data on the effectiveness of prophylaxis with RELENZA in a household setting when initiated more than 1.5 days after the onset of signs or symptoms in the index case.
Community Outbreaks
- The recommended dose of RELENZA for prophylaxis of influenza in adults and adolescents in a community setting is 10 mg once daily for 28 days.
- The dose should be administered at approximately the same time each day.
- There are no data on the effectiveness of prophylaxis with RELENZA in a community outbreak when initiated more than 5 days after the outbreak was identified in the community.
- The safety and effectiveness of prophylaxis with RELENZA have not been evaluated for longer than 28 days’ duration.
-
3 DOSAGE FORMS AND STRENGTHS
Blister for oral inhalation: 5 mg. Four 5-mg blisters of powder on a ROTADISK for oral inhalation via DISKHALER. Packaged in carton containing 5 ROTADISKs (total of 10 doses) and 1 DISKHALER inhalation device [see How Supplied/Storage and Handling (16)].
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Bronchospasm
RELENZA is not recommended for treatment or prophylaxis of influenza in individuals with underlying airways disease (such as asthma or chronic obstructive pulmonary disease).
Serious cases of bronchospasm, including fatalities, have been reported during treatment with RELENZA in patients with and without underlying airways disease. Many of these cases were reported during postmarketing and causality was difficult to assess.
RELENZA should be discontinued in any patient who develops bronchospasm or decline in respiratory function; immediate treatment and hospitalization may be required.
Some patients without prior pulmonary disease may also have respiratory abnormalities from acute respiratory infection that could resemble adverse drug reactions or increase patient vulnerability to adverse drug reactions.
Bronchospasm was documented following administration of zanamivir in 1 of 13 subjects with mild or moderate asthma (but without acute influenza‑like illness) in a Phase 1 trial. In a Phase 3 trial in subjects with acute influenza‑like illness superimposed on underlying asthma or chronic obstructive pulmonary disease, 10% (24 of 244) of subjects on zanamivir and 9% (22 of 237) on placebo experienced a greater than 20% decline in FEV1 following treatment for 5 days.
If use of RELENZA is considered for a patient with underlying airways disease, the potential risks and benefits should be carefully weighed. If a decision is made to prescribe RELENZA for such a patient, this should be done only under conditions of careful monitoring of respiratory function, close observation, and appropriate supportive care, including availability of fast‑acting bronchodilators.
5.2 Allergic Reactions
Allergic-like reactions, including oropharyngeal edema, serious skin rashes, and anaphylaxis have been reported in postmarketing experience with RELENZA. RELENZA should be stopped and appropriate treatment instituted if an allergic reaction occurs or is suspected.
5.3 Neuropsychiatric Events
Influenza can be associated with a variety of neurologic and behavioral symptoms which can include events such as seizures, hallucinations, delirium, and abnormal behavior, in some cases resulting in fatal outcomes. These events may occur in the setting of encephalitis or encephalopathy but can occur without obvious severe disease.
There have been postmarketing reports of delirium and abnormal behavior leading to injury in patients with influenza who were receiving neuraminidase inhibitors (NAIs), including RELENZA. Because these events were reported voluntarily during clinical practice, estimates of frequency cannot be made, but they appear to be uncommon based on usage data for RELENZA. These events were reported primarily among pediatric patients and often had an abrupt onset and rapid resolution. The contribution of RELENZA to these events has not been established. Patients with influenza should be closely monitored for signs of abnormal behavior. If neuropsychiatric symptoms occur, the risks and benefits of continuing treatment should be evaluated for each patient.
5.4 Limitations of Populations Studied
Safety and efficacy have not been demonstrated in patients with high-risk underlying medical conditions. No information is available regarding treatment of influenza in patients with any medical condition sufficiently severe or unstable to be considered at imminent risk of requiring inpatient management.
5.5 Bacterial Infections
Serious bacterial infections may begin with influenza-like symptoms or may coexist with or occur as complications during the course of influenza. RELENZA has not been shown to prevent such complications.
5.6 Importance of Proper Route of Administration
RELENZA inhalation powder must not be made into an extemporaneous solution for administration by nebulization or mechanical ventilation. There have been reports of hospitalized patients with influenza who received a solution made with RELENZA inhalation powder administered by nebulization or mechanical ventilation, including a fatal case where it was reported that the lactose in this formulation obstructed the proper functioning of the equipment. RELENZA inhalation powder must only be administered using the device provided [see Dosage and Administration (2.1)].
5.7 Importance of Proper Use of DISKHALER
Effective and safe use of RELENZA requires proper use of the DISKHALER to inhale the drug. Prescribers should carefully evaluate the ability of young children to use the delivery system if use of RELENZA is considered [see Use in Specific Populations (8.4)].
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Bronchospasm [see Warnings and Precautions (5.1)].
- Safety information in patients with underlying airways disease [see Warnings and Precautions (5.1)].
- Allergic-like reactions [see Warnings and Precautions (5.2)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The placebo used in clinical trials consisted of inhaled lactose powder, which is also the vehicle for the active drug; therefore, some adverse events occurring at similar frequencies in different treatment groups could be related to lactose vehicle inhalation.
Treatment of Influenza
Clinical Trials in Adults and Adolescents: Adverse events that occurred with an incidence greater than or equal to 1.5% in treatment trials are listed in Table 1. This table shows adverse events occurring in subjects aged 12 years and older receiving RELENZA 10 mg inhaled twice daily, RELENZA in all inhalation regimens, and placebo inhaled twice daily (where placebo consisted of the same lactose vehicle used in RELENZA).
Table 1. Summary of Adverse Events ≥1.5% Incidence during Treatment in Adults and Adolescents a Includes trials where RELENZA was administered intranasally (6.4 mg 2 to 4 times per day in addition to inhaled preparation) and/or inhaled more frequently (q.i.d.) than the currently recommended dose. Adverse Event
RELENZA
Placebo
(Lactose Vehicle)
(n = 1,520)
10 mg b.i.d. Inhaled
(n = 1,132)
All Dosing Regimensa
(n = 2,289)
Body as a whole
Headaches
2%
2%
3%
Digestive
Diarrhea
3%
3%
4%
Nausea
3%
3%
3%
Vomiting
1%
1%
2%
Respiratory
Nasal signs and symptoms
2%
3%
3%
Bronchitis
2%
2%
3%
Cough
2%
2%
3%
Sinusitis
3%
2%
2%
Ear, nose, and throat infections
2%
1%
2%
Nervous system
Dizziness
2%
1%
<1%
Additional adverse reactions occurring in less than 1.5% of subjects receiving RELENZA included malaise, fatigue, fever, abdominal pain, myalgia, arthralgia, and urticaria.
The most frequent laboratory abnormalities in Phase 3 treatment trials included elevations of liver enzymes and CPK, lymphopenia, and neutropenia. These were reported in similar proportions of zanamivir and lactose vehicle placebo recipients with acute influenza‑like illness.
Clinical Trials in Pediatric Subjects: Adverse events that occurred with an incidence greater than or equal to 1.5% in children receiving treatment doses of RELENZA in 2 Phase 3 trials are listed in Table 2. This table shows adverse events occurring in pediatric subjects aged 5 to 12 years receiving RELENZA 10 mg inhaled twice daily and placebo inhaled twice daily (where placebo consisted of the same lactose vehicle used in RELENZA).
Table 2. Summary of Adverse Events ≥1.5% Incidence during Treatment in Pediatric Subjectsa a Includes a subset of subjects receiving RELENZA for treatment of influenza in a prophylaxis trial. Adverse Event
RELENZA
10 mg b.i.d. Inhaled
(n = 291)
Placebo
(Lactose Vehicle)
(n = 318)
Respiratory
Ear, nose, and throat infections
5%
5%
Ear, nose, and throat hemorrhage
<1%
2%
Asthma
<1%
2%
Cough
<1%
2%
Digestive
Vomiting
2%
3%
Diarrhea
2%
2%
Nausea
<1%
2%
In 1 of the 2 trials described in Table 2, some additional information is available from children (aged 5 to 12 years) without acute influenza-like illness who received an investigational prophylaxis regimen of RELENZA; 132 children received RELENZA and 145 children received placebo. Among these children, nasal signs and symptoms (zanamivir 20%, placebo 9%), cough (zanamivir 16%, placebo 8%), and throat/tonsil discomfort and pain (zanamivir 11%, placebo 6%) were reported more frequently with RELENZA than placebo. In a subset with chronic pulmonary disease, lower respiratory adverse events (described as asthma, cough, or viral respiratory infections which could include influenza-like symptoms) were reported in 7 of 7 zanamivir recipients and 5 of 12 placebo recipients.
Prophylaxis of Influenza
Family/Household Prophylaxis Trials: Adverse events that occurred with an incidence of greater than or equal to 1.5% in the 2 prophylaxis trials are listed in Table 3. This table shows adverse events occurring in subjects aged 5 years and older receiving RELENZA 10 mg inhaled once daily for 10 days.
Table 3. Summary of Adverse Events ≥1.5% Incidence during 10-Day Prophylaxis Trials in Adults, Adolescents, and Childrena a In prophylaxis trials, symptoms associated with influenza-like illness were captured as adverse events; subjects were enrolled during a winter respiratory season during which time any symptoms that occurred were captured as adverse events. Adverse Event
Contact Cases
RELENZA
(n = 1,068)
Placebo
(n = 1,059)
Lower respiratory
Viral respiratory infections
13%
19%
Cough
7%
9%
Neurologic
Headaches
13%
14%
Ear, nose, and throat
Nasal signs and symptoms
12%
12%
Throat and tonsil discomfort and pain
8%
9%
Nasal inflammation
1%
2%
Musculoskeletal
Muscle pain
3%
3%
Endocrine and metabolic
Feeding problems (decreased or increased appetite and anorexia)
2%
2%
Gastrointestinal
Nausea and vomiting
1%
2%
Non-site specific
Malaise and fatigue
5%
5%
Temperature regulation disturbances (fever and/or chills)
5%
4%
Community Prophylaxis Trials: Adverse events that occurred with an incidence of greater than or equal to 1.5% in 2 prophylaxis trials are listed in Table 4. This table shows adverse events occurring in subjects aged 5 years and older receiving RELENZA 10 mg inhaled once daily for 28 days.
Table 4. Summary of Adverse Events ≥1.5% Incidence during 28-Day Prophylaxis Trials in Adults, Adolescents, and Childrena a In prophylaxis trials, symptoms associated with influenza-like illness were captured as adverse events; subjects were enrolled during a winter respiratory season during which time any symptoms that occurred were captured as adverse events. Adverse Event
RELENZA
(n = 2,231)
Placebo
(n = 2,239)
Neurologic
Headaches
24%
26%
Ear, nose, and throat
Throat and tonsil discomfort and pain
19%
20%
Nasal signs and symptoms
12%
13%
Ear, nose, and throat infections
2%
2%
Lower respiratory
Cough
17%
18%
Viral respiratory infections
3%
4%
Musculoskeletal
Muscle pain
8%
8%
Musculoskeletal pain
6%
6%
Arthralgia and articular rheumatism
2%
<1%
Endocrine and metabolic
Feeding problems (decreased or increased appetite and anorexia)
4%
4%
Gastrointestinal
Nausea and vomiting
2%
3%
Diarrhea
2%
2%
Non-site specific
Temperature regulation disturbances (fever and/or chills)
9%
10%
Malaise and fatigue
8%
8%
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of RELENZA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Allergic Reactions
Allergic or allergic-like reaction, including oropharyngeal edema [see Warnings and Precautions (5.2)].
Psychiatric
Delirium, including symptoms such as altered level of consciousness, confusion, abnormal behavior, delusions, hallucinations, agitation, anxiety, nightmares [see Warnings and Precautions (5.3)].
Cardiac
Arrhythmias, syncope.
Neurologic
Seizures. Vasovagal-like episodes have been reported shortly following inhalation of zanamivir.
Respiratory
Bronchospasm, dyspnea [see Warnings and Precautions (5.1)].
Skin
Facial edema; rash, including serious cutaneous reactions (e.g., erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis); urticaria [see Warnings and Precautions (5.2)].
-
7 DRUG INTERACTIONS
The concurrent use of RELENZA with live attenuated influenza vaccine (LAIV) intranasal has not been evaluated. However, because of potential interference between these products, LAIV should not be administered within 2 weeks before or 48 hours after administration of RELENZA, unless medically indicated. The concern about possible interference arises from the potential for antiviral drugs to inhibit replication of live vaccine virus.
Trivalent inactivated influenza vaccine can be administered at any time relative to use of RELENZA [see Microbiology (12.4)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from published studies suggest that use of RELENZA during pregnancy is not associated with an increased risk of birth defects or adverse maternal or fetal outcomes. However, these studies are limited by their small sample sizes, which preclude a definitive assessment of the risk (see Data). There are risks to the mother and fetus associated with influenza infection in pregnancy (see Clinical Considerations). In animal reproduction studies, no adverse developmental effects were observed with intravenous or subcutaneous administration of zanamivir at exposures 300 and 150 times, respectively, the systemic exposure at the maximum recommended human inhalation dose (MRHID) of 10 mg twice daily (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk: Pregnant women are at higher risk of severe complications from influenza, which may lead to adverse pregnancy and/or fetal outcomes, including maternal death, stillbirths, birth defects, preterm delivery, low birth weight, and small for gestational age.
Data
Human Data: A study of population-based registers from Denmark, Norway, Sweden, and France reported outcomes of 5,824 pregnant women who filled a prescription for an NAI compared with outcomes in unexposed pregnant women in the general population. This study included 1,560 women who filled a prescription for zanamivir (including 321 first trimester exposures). Although no specific analyses were conducted for zanamivir, exposure to the NAI class in utero was not associated with major birth defects, preterm birth, low birth weight, small for gestational age, still birth, neonatal morbidity, or neonatal mortality.
A few published studies compared outcomes of pregnant women exposed to zanamivir with outcomes in various comparator cohorts. These studies suggested no increased risk of major birth defects, preterm birth, or low birth weight. Sample sizes in these studies ranged from 50 to 180 pregnant women exposed to zanamivir, including 15 to 44 women exposed in the first trimester. Limitations of available studies include the lack of specific analyses for zanamivir, possible exposure and outcome misclassification, and small sample sizes. These limitations preclude a definitive assessment of the risk.
Animal Data: Zanamivir was administered intravenously to pregnant rats and rabbits at 1, 9, or 90 mg/kg/day during organogenesis (gestation Day 6 to 15 [rat] and 7 to 19 [rabbit]). No adverse maternal or embryo-fetal effects were observed up to the highest intravenous dose of zanamivir (90 mg/kg/day), resulting in systemic drug exposure (AUC) estimated from both rats and rabbits, 300 times the exposure at the MRHID. In a separate study, zanamivir was administered subcutaneously to pregnant rats at 3, 27, and 240 mg/kg/day in three divided doses during organogenesis (gestation Day 7 to 17). An increased incidence of skeletal and visceral alterations and variants was observed at the high dose (240 mg/kg/day). No adverse maternal or embryo-fetal effects were observed at the middle dose (27 mg/kg/day), resulting in systemic drug exposure (AUC) 150 times the exposure at the MRHID.
In prenatal and postnatal development studies in rats, zanamivir was administered intravenously at 1, 9, or 90 mg/kg/day during organogenesis (gestation Day 0 to 19) or from late gestation through delivery and lactation (gestation Day 16 to post-partum/lactation Day 21). No significant effects were observed in the offspring at systemic drug exposures (AUC) estimated to be 300 times the exposure at the MRHID.
Zanamivir distributed across the placenta in pregnant rats and rabbits, with less than 0.04% of the administered maternal dose being recovered from the fetus on gestation Day 12.
8.2 Lactation
Risk Summary
There are no data on the presence of zanamivir in human milk or the effects on milk production. There are data from adults that have shown low oral bioavailability of zanamivir. Limited data from postmarketing case reports have not suggested a safety concern in infants exposed to breast milk of mothers using RELENZA. Zanamivir was present in the milk of lactating rats without effect on nursing pups (see Data). The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for RELENZA and any potential adverse effects on the breastfed child from RELENZA or the underlying maternal condition.
Data
In a lactation study, zanamivir was excreted in the milk of lactating rats administered zanamivir intravenously (10 mg/kg) on post-partum/lactation Day 10, with peak milk concentrations of approximately 10% that of maternal plasma concentrations occurring 30 minutes post-dose. No effects of zanamivir on growth and postnatal development were observed in nursing pups at the highest intravenous dose tested in rats. Maternal systemic exposure (AUC) of zanamivir was approximately 300 times the exposure in humans at the MRHID.
8.4 Pediatric Use
Treatment of Influenza
Safety and effectiveness of RELENZA for treatment of influenza have not been assessed in pediatric patients younger than 7 years, but were studied in a Phase 3 treatment trial in pediatric subjects, where 471 children aged 5 to 12 years received zanamivir or placebo [see Clinical Studies (14.1)]. Adolescents were included in the 3 principal Phase 3 adult treatment trials. In these trials, 67 patients were aged 12 to 16 years. No definite differences in safety and efficacy were observed between these adolescent patients and young adults.
In a Phase 1 trial of 16 children aged 6 to 12 years with signs and symptoms of respiratory disease, 4 did not produce a measurable peak inspiratory flow rate (PIFR) through the DISKHALER (3 with no adequate inhalation on request, 1 with missing data), 9 had measurable PIFR on each of 2 inhalations, and 3 achieved measurable PIFR on only 1 of 2 inhalations. Neither of two 6-year-olds and one of two 7-year-olds produced measurable PIFR. Overall, 8 of the 16 children (including all those younger than 8 years) either did not produce measurable inspiratory flow through the DISKHALER or produced peak inspiratory flow rates below the 60 L/minute considered optimal for the device under standardized in vitro testing; lack of measurable flow rate was related to low or undetectable serum concentrations [see Clinical Pharmacology (12.3), Clinical Studies (14.1)]. Prescribers should carefully evaluate the ability of young children to use the delivery system if prescription of RELENZA is considered.
Prophylaxis of Influenza
The safety and effectiveness of RELENZA for prophylaxis of influenza have been studied in 4 Phase 3 trials where 273 children aged 5 to 11 years and 239 adolescents aged 12 to 16 years received RELENZA. No differences in safety and effectiveness were observed between pediatric and adult subjects [see Clinical Studies (14.2)].
8.5 Geriatric Use
Of the total number of subjects in 6 clinical trials of RELENZA for treatment of influenza, 59 subjects were aged 65 years and older, while 24 subjects were aged 75 years and older. Of the total number of subjects in 4 clinical trials of RELENZA for prophylaxis of influenza in households and community settings, 954 subjects were aged 65 years and older, while 347 subjects were aged 75 years and older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger subjects, but greater sensitivity of some older individuals cannot be ruled out. Elderly patients may need assistance with use of the device.
In 2 additional trials of RELENZA for prophylaxis of influenza in the nursing home setting, efficacy was not demonstrated [see Indications and Usage (1.3)].
8.6 Renal Impairment
Safety and efficacy have not been documented in the presence of severe renal insufficiency. Due to the low systemic bioavailability of zanamivir following oral inhalation, no dosage adjustments are necessary in patients with renal impairment. However, the potential for drug accumulation should be considered.
-
10 OVERDOSAGE
Reports of overdosage from administration of RELENZA have been received during postmarketing experience. The reported clinical signs or symptoms were similar to those observed with therapeutic doses of RELENZA and the underlying disease.
As zanamivir has a low molecular weight, low protein binding, and small volume of distribution, it is expected to be removed by hemodialysis.
-
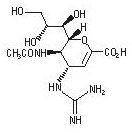
11 DESCRIPTION
The active component of RELENZA is zanamivir. The chemical name of zanamivir is 5-(acetylamino)-4-[(aminoiminomethyl)-amino]-2,6-anhydro-3,4,5-trideoxy-D-glycero-D-galacto-non-2-enonic acid. It has a molecular formula of C12H20N4O7 and a molecular weight of 332.3. It has the following structural formula:
Zanamivir is a white to off-white powder for oral inhalation with a solubility of approximately 18 mg/mL in water at 20°C.
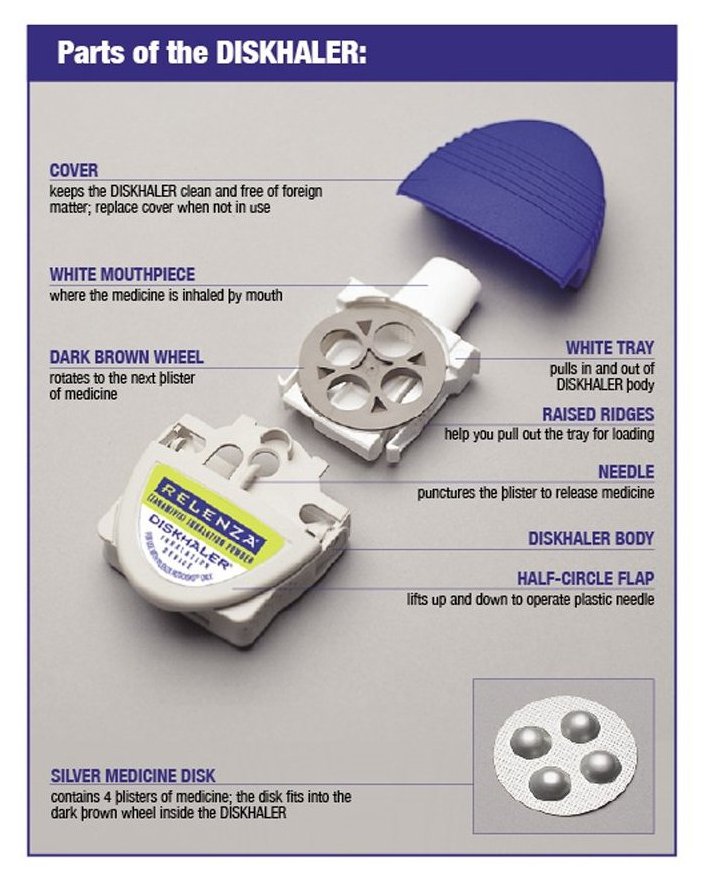
RELENZA is for administration to the respiratory tract by oral inhalation only. Each RELENZA ROTADISK contains 4 regularly spaced double-foil blisters with each blister containing a powder mixture of 5 mg of zanamivir and 20 mg of lactose (which contains milk proteins). The contents of each blister are inhaled using a specially designed breath-activated plastic device for inhaling powder called the DISKHALER. After a RELENZA ROTADISK is loaded into the DISKHALER, a blister that contains medication is pierced and the zanamivir is dispersed into the air stream created when the patient inhales through the mouthpiece. The amount of drug delivered to the respiratory tract will depend on patient factors such as inspiratory flow. Under standardized in vitro testing, RELENZA ROTADISK delivers 4 mg of zanamivir from the DISKHALER device when tested at a pressure drop of 3 kPa (corresponding to a flow rate of about 62 to 65 L/minute) for 3 seconds.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Zanamivir is an antiviral drug with activity against influenza virus [see Microbiology (12.4)].
12.2 Pharmacodynamics
Cardiac Electrophysiology
In a thorough QT/QTc study in 40 healthy subjects, intravenous zanamivir 600 mg and 1,200 mg (60 and 120 times the dose in RELENZA) had no effect on the QTc interval and did not prolong the PR interval.
12.3 Pharmacokinetics
Absorption and Bioavailability
Pharmacokinetic studies of orally inhaled zanamivir indicate that approximately 4% to 17% of the inhaled dose is systemically absorbed. The peak serum concentrations ranged from 17 to 142 ng/mL within 1 to 2 hours following a 10‑mg dose. The area under the serum concentration versus time curve (AUC∞) ranged from 111 to 1,364 nghour/mL.
Distribution
Zanamivir plasma protein binding is less than 10% and the blood to plasma ratio is 0.56. The volume of distribution is approximately 16 L. The 2 major sites of deposition are the oropharynx and the lungs (mean 77.6% and 13.2%, respectively).
Elimination
The serum half‑life of zanamivir following administration by oral inhalation ranges from 2.5 to 5.1 hours.
Metabolism: No metabolites have been detected in humans.
Excretion: Zanamivir is eliminated by renal filtration with excretion of a single dose completed within 24 hours. Total clearance ranges from 2.5 to 10.9 L/hour. Unabsorbed drug is excreted in the feces.
Specific Populations
Patients with Hepatic Impairment: The pharmacokinetics of zanamivir have not been studied in patients with impaired hepatic function.
Patients with Renal Impairment: After a single intravenous dose of 4 mg or 2 mg of zanamivir in volunteers with renal impairment determined by the Cockcroft and Gault method, significant increases in systemic exposure were observed (Table 5).
Table 5. Zanamivir Exposure in Subjects with Renal Impairment Compared with Healthy Subjects a Geometric mean (95% confidence interval)
Cmax = Maximum serum concentration; AUC0-inf = Area under the serum concentration-time curve from zero to infinity; CrCl = Creatinine Clearance.CrCl >70 mL/min
(n = 7)CrCl = 25-70 mL/min
(n = 5)CrCl <25 mL/min
(n = 5)Cmax (ng/mL)a
257 (182, 363)
316 (234, 427)
202 (141, 289)
AUC0-inf (ng∙h/mL)a
737 (567, 957)
1,519 (1181, 1954)
2,587 (1514, 4419)
Pediatric Patients: The pharmacokinetics of zanamivir were evaluated in pediatric subjects with signs and symptoms of respiratory illness. Sixteen subjects, aged 6 to 12 years, received a single dose of 10 mg zanamivir dry powder via DISKHALER. Five subjects had either undetectable zanamivir serum concentrations or had low drug concentrations (8.32 to 10.38 ng/mL) that were not detectable after 1.5 hours. Eleven subjects had Cmax median values of 43 ng/mL (range: 15 to 74) and AUC∞ median values of 167 nghour/mL (range: 58 to 279). Low or undetectable serum concentrations were related to lack of measurable PIFR in individual subjects [see Use in Specific Populations (8.4), Clinical Studies (14.1)].
Geriatric Patients: The pharmacokinetics of zanamivir have not been studied in subjects older than 65 years [see Use in Specific Populations (8.5)].
Gender, Race, and Weight: There were no clinically significant pharmacokinetic changes associated with gender, race, or weight.
Drug Interaction Studies
Zanamivir is not a substrate of cytochrome P450 (CYP) isoenzymes or P-glycoprotein (P-gp), does not inhibit renal transporters OAT1-4, OCT1-3, or URAT1, and does not inhibit CYP isoenzymes CYP1A1/2, 2A6, 2C9, 2C19, 2D6, 2E1, and 3A4 in human liver microsomes.
12.4 Microbiology
Mechanism of Action
Zanamivir is an inhibitor of influenza virus neuraminidase, an enzyme that releases viral particles from the plasma membrane of infected cells and promotes virus spread in the respiratory tract. In neuraminidase inhibition assays, zanamivir is active against all neuraminidase (NA) enzyme subtypes N1 to N9. The median baseline 50% neuraminidase inhibitory concentrations (IC50) of zanamivir for influenza type A subtype H1N1, influenza type A subtype H3N2, and influenza type B viruses were 0.85 nM (n = 31; range: 0.45 to 1.35 nM), 1.01 nM (n = 32; range: 0.33 to 2.04 nM), and 3.86 nM (n = 16; range: 2.02 to 5.77 nM), respectively, in a chemiluminescent assay. Amino acid substitutions have been found in the neuraminidase active site that reduce virus susceptibility to zanamivir (see Resistance).
Antiviral Activity
Zanamivir has been demonstrated to inhibit laboratory and clinical isolates of influenza virus in cell culture. The median EC50 values of zanamivir in cell culture assays for influenza type A (subtypes H1N1 and H3N2) and influenza type B viruses were 210 nM (70 ng/mL) (n = 10; range: 1 to 16,000 nM), 14 nM (4.7 ng/mL) (n = 12; range: 1 to 1,700 nM), and 18 nM (6 ng/mL) (n = 9; range: 3 to 1,300 nM), respectively. The relationship between the cell culture inhibition of influenza virus by zanamivir and the inhibition of influenza virus replication in humans has not been established.
Resistance
Cell Culture: Influenza viruses with reduced susceptibility to zanamivir have been selected in cell culture by multiple passages of the virus in the presence of increasing concentrations of the drug. Reduced susceptibility of influenza virus to inhibition by zanamivir may be conferred by amino acid substitutions in the viral NA and/or hemagglutinin (HA) proteins (Table 6).
Table 6: Amino Acid Substitutions Selected by Zanamivir in Cell Culture Studies a Numbering based on A/California/04/2009.
b Numbering based on A/Texas/50/2012.
c Numbering based on B/Massachusetts/02/2012.
d HA numbering begins after the predicted signal peptide.Protein
Type/Subtype
A/H1N1a
A/H3N2b
Bc
NA
D199G
T148I
E117D/G
HAd
G155E, A197T
--
V90A, N145S, N150S, Q226K, L239Q
Clinical: Influenza viruses with amino acid substitutions and deletions associated with reduced susceptibility to zanamivir have been identified in patients treated with zanamivir and in circulating viruses from untreated individuals. Genetic analysis of these viruses showed that the reduced susceptibility to zanamivir is associated with mutations that result in amino acid changes in the viral NA or viral HA or both (see Table 7). The clinical impact of substitutions that reduce virus susceptibility to zanamivir is variable and may be strain-dependent.
Table 7: Amino Acid Substitutions Observed in Virus from Humans and Associated with Reduced Susceptibility to Zanamivir a Numbering based on A/California/04/2009.
b Numbering based on A/Texas/50/2012.
c Numbering based on B/Massachusetts/02/2012.
d Emerged in patients treated with zanamivir.
e HA numbering begins after the predicted signal peptide.Protein
Type/Subtype
A/H1N1a
A/H3N2b
Bc
NA
N70S, E119Dd/Gd/Kd, H126N, Q136K, D151G+H275Y, Y155H, I223K/Rd, S247I/R, G249R, I267V, Q313R, N325K, R368Gd G370Dd, I427T, N434Sd, G460S
Q136K, D151A/G/V, V165I+H274N, G248R+K249E, N294Sd, T325Id
G104E, E105K, T106L+P165L, H134N, Q138R, P139S, G140R, R150Kd, K186R+I262T, D197E/Y, A200T, I221Td, G243S, A245T, S249G, R374K, G407S
HAe
L151Pd, V152I, S162N, S183P, A197T, D222G
R142Gd, N225D
T198Id
Zoonotic Viruses: Substitutions conferring reduced susceptibility to zanamivir have been observed in viruses with zoonotic potential (Table 8). The clinical impact of reduced susceptibility in these viruses is unknown, and the effects of specific substitutions on virus susceptibility to zanamivir may be strain-dependent.
Table 8: Amino Acid Substitutions Observed in Avian Influenza Viruses with Zoonotic Potential and Associated with Reduced Susceptibility to Zanamivir a Numbering based on A/Vietnam/1203/2004.
b Numbering based on A/Anhui/1/2013.
c Selected in cell culture or animal models of influenza.
d Observed in virus isolated from humans.Protein
Type/Subtype
A/H5N1a (N2 numbering)
A/H7N9b (N2 numbering)
NA
V96A (116), E99A/Gc (119), Q116Lc (136), V129Ad (149), D179G (198)
E115Vd (119), R148Kd (152), I219K/Rd (222), R289Kd (292)
Circulating resistant variants may change from season to season. Prescribers should consider available information from the U.S. Centers for Disease Control and Prevention regarding influenza virus drug susceptibility patterns and treatment effects when deciding whether to use zanamivir.
Cross-Resistance
Cross-resistance between zanamivir and oseltamivir or peramivir has been observed in neuraminidase inhibition assays and cell culture assays. The NA amino acid substitutions that have been shown to result in reduced susceptibility to zanamivir and either oseltamivir or peramivir are summarized in Table 9. HA substitutions associated with reduced susceptibility to zanamivir may reduce susceptibility to all NAIs. The clinical impact of substitutions associated with reduced susceptibility to zanamivir and other NAIs is variable and may be strain-dependent.
Table 9: Summary of Neuraminidase Amino Acid Substitutions with Cross-Resistance between Zanamivir and Oseltamivir or Peramivir in Susceptibility Assays a Numbering based on A/California/04/2009.
b Numbering based on A/Texas/50/2012.
c Numbering based on B/Massachusetts/02/2012.
d Deletion of amino acids SASG.Neuraminidase Inhibitor
Type/Subtype
A/H1N1a
A/H3N2b
Bc
Oseltamivir
E119D/G, H126N, D151G+H275Y, R152K, Y155H, D199G, I223R/K, S247R, I267V, Q313R, N325K, R368K, I427T
E119I/V, D151V, ∆245-248d, R292K, N294S
G104E, E105K, E117D/G, P139S, G140R, R150K, D197E/N/Y, A200T, I221T, A245T, R374K, G407S
Peramivir
E119D/G, H126N, Q136K, D151G+H275Y, D199G, I223R/K, S247R, I427T
E119I/V, Q136K, D151A/G/V, R292K
G104E, E105K, E117G, H134N, Q138R, P139S, G140R, D197E/N/Y, I221T, R374K, G407S
Influenza Vaccine Interaction Trial
An interaction trial (n = 138) was conducted to evaluate the effects of zanamivir (10 mg once daily) on the serological response to a single dose of trivalent inactivated influenza vaccine, as measured by hemagglutination inhibition titers. There was no difference in hemagglutination inhibition antibody titers at 2 weeks and 4 weeks after vaccine administration between zanamivir and placebo recipients.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In 2-year carcinogenicity studies conducted in rats and mice using a powder formulation administered through inhalation, zanamivir induced no statistically significant increases in tumors over controls. The maximum daily exposures in rats and mice were approximately 23 to 25 and 20 to 22 times, respectively, greater than those in humans at the proposed clinical dose based on AUC comparisons.
Mutagenesis
Zanamivir was not mutagenic in in vitro and in vivo genotoxicity assays which included bacterial mutation assays in S. typhimurium and E. coli, mammalian mutation assays in mouse lymphoma, chromosomal aberration assays in human peripheral blood lymphocytes, and the in vivo mouse bone marrow micronucleus assay.
Impairment of Fertility
Zanamivir did not impair mating or fertility of male or female rats and did not affect the sperm of treated male rats dosed for 10 weeks prior to mating and throughout mating, gestation/lactation, and shortly after weaning, and female rats dosed for 3 weeks prior to mating through organogenesis or lactation, at IV doses 1, 9, and 90 mg/kg/day, resulting in systemic drug exposures (AUC) estimated to be up to 300 times higher than the MRHID.
-
14 CLINICAL STUDIES
14.1 Treatment of Influenza
Adults and Adolescents
The efficacy of RELENZA 10 mg inhaled twice daily for 5 days in the treatment of influenza has been evaluated in placebo-controlled trials conducted in North America, the Southern Hemisphere, and Europe during their respective influenza seasons. The magnitude of treatment effect varied between trials, with possible relationships to population-related factors including amount of symptomatic relief medication used.
Populations Studied: The principal Phase 3 trials enrolled 1,588 subjects aged 12 years and older (median age 34 years, 49% male, 91% Caucasian), with uncomplicated influenza-like illness within 2 days of symptom onset. Influenza was confirmed by culture, hemagglutination inhibition antibodies, or investigational direct tests. Of 1,164 subjects with confirmed influenza, 89% had influenza A and 11% had influenza B. These trials served as the principal basis for efficacy evaluation, with more limited Phase 2 studies providing supporting information where necessary. Following randomization to either zanamivir or placebo (inhaled lactose vehicle), all subjects received instruction and supervision by a healthcare professional for the initial dose.
Principal Results: The definition of time to improvement in major symptoms of influenza included no fever and self-assessment of “none” or “mild” for headache, myalgia, cough, and sore throat. A Phase 2 and a Phase 3 trial conducted in North America (total of over 600 influenza-positive subjects) suggested up to 1 day of shortening of median time to this defined improvement in symptoms in subjects receiving zanamivir compared with placebo, although statistical significance was not reached in either of these trials. In a trial conducted in the Southern Hemisphere (321 influenza-positive subjects), a 1.5-day difference in median time to symptom improvement was observed. Additional evidence of efficacy was provided by the European trial.
Other Findings: There was no consistent difference in treatment effect in subjects with influenza A compared with influenza B; however, these trials enrolled smaller numbers of subjects with influenza B and thus provided less evidence in support of efficacy in influenza B.
In general, subjects with lower temperature (e.g., 38.2°C or less) or investigator-rated as having less severe symptoms at entry derived less benefit from therapy.
No consistent treatment effect was demonstrated in subjects with underlying chronic medical conditions, including respiratory or cardiovascular disease [see Warnings and Precautions (5.4)].
No consistent differences in rate of development of complications were observed between treatment groups.
Some fluctuation of symptoms was observed after the primary trial endpoint in both treatment groups.
Pediatric Patients
The efficacy of RELENZA 10 mg inhaled twice daily for 5 days in the treatment of influenza in pediatric patients has been evaluated in a placebo-controlled trial conducted in North America and Europe, enrolling 471 subjects, aged 5 to 12 years (55% male, 90% Caucasian), within 36 hours of symptom onset. Of 346 subjects with confirmed influenza, 65% had influenza A and 35% had influenza B. The definition of time to improvement included no fever and parental assessment of no or mild cough and absent/minimal muscle and joint aches or pains, sore throat, chills/feverishness, and headache. Median time to symptom improvement was 1 day shorter in subjects receiving zanamivir compared with placebo. No consistent differences in rate of development of complications were observed between treatment groups. Some fluctuation of symptoms was observed after the primary trial endpoint in both treatment groups.
Although this trial was designed to enroll children aged 5 to 12 years, the product is indicated only for children aged 7 years and older. This evaluation is based on the combination of lower estimates of treatment effect in 5- and 6-year-olds compared with the overall trial population, and evidence of inadequate inhalation through the DISKHALER in a pharmacokinetic trial [see Use in Specific Populations (8.4), Clinical Pharmacology (12.3)].
14.2 Prophylaxis of Influenza
The efficacy of RELENZA in preventing naturally occurring influenza illness has been demonstrated in 2 post-exposure prophylaxis trials in households and 2 seasonal prophylaxis trials during community outbreaks of influenza. The primary efficacy endpoint in these trials was the incidence of symptomatic, laboratory-confirmed influenza, defined as the presence of 2 or more of the following symptoms: oral temperature greater than or equal to 100°F/37.8°C or feverishness, cough, headache, sore throat, and myalgia; and laboratory confirmation of influenza A or B by culture, PCR, or seroconversion (defined as a 4-fold increase in convalescent antibody titer from baseline).
Household Prophylaxis Trials
Two trials assessed post-exposure prophylaxis in household contacts of an index case. Within 1.5 days of onset of symptoms in an index case, each household (including all family members aged 5 years and older) was randomized to RELENZA 10 mg inhaled once daily or placebo inhaled once daily for 10 days. In the first trial only, each index case was randomized to RELENZA 10 mg inhaled twice daily for 5 days or inhaled placebo twice daily for 5 days. In this trial, the proportion of households with at least 1 new case of symptomatic laboratory-confirmed influenza was reduced from 19.0% (32 of 168 households) for the placebo group to 4.1% (7 of 169 households) for the group receiving RELENZA.
In the second trial, index cases were not treated. The incidence of symptomatic laboratory-confirmed influenza was reduced from 19.0% (46 of 242 households) for the placebo group to 4.1% (10 of 245 households) for the group receiving RELENZA.
Seasonal Prophylaxis Trials
Two seasonal prophylaxis trials assessed RELENZA 10 mg inhaled once daily versus placebo inhaled once daily for 28 days during community outbreaks. The first trial enrolled subjects aged 18 years or older (mean age: 29 years) from 2 university communities. The majority of subjects were unvaccinated (86%). In this trial, the incidence of symptomatic laboratory-confirmed influenza was reduced from 6.1% (34 of 554) for the placebo group to 2.0% (11 of 553) for the group receiving RELENZA.
The second seasonal prophylaxis trial enrolled subjects aged 12 to 94 years (mean age: 60 years) with 56% of them older than 65 years. Sixty-seven percent of the subjects were vaccinated. In this trial, the incidence of symptomatic laboratory-confirmed influenza was reduced from 1.4% (23 of 1,685) for the placebo group to 0.2% (4 of 1,678) for the group receiving RELENZA.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
RELENZA is supplied in a circular double-foil pack (a ROTADISK) containing 4 blisters of the drug. Five ROTADISKs are packaged in a white polypropylene tube. The tube is packaged in a carton with 1 blue and gray DISKHALER inhalation device (NDC: 0173-0681-01).
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) (see USP Controlled Room Temperature). Keep out of reach of children. Do not puncture any RELENZA ROTADISK blister until taking a dose using the DISKHALER.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Bronchospasm
Inform patients of the risk of bronchospasm, especially in the setting of underlying airways disease, and advise patients to stop RELENZA and contact their healthcare provider if they experience increased respiratory symptoms during treatment such as worsening wheezing, shortness of breath, or other signs or symptoms of bronchospasm [see Warnings and Precautions (5.1)]. If a decision is made to prescribe RELENZA for a patient with asthma or chronic obstructive pulmonary disease, the patient should be made aware of the risks and should have a fast-acting bronchodilator available.
Concomitant Bronchodilator Use
Patients scheduled to take inhaled bronchodilators at the same time as RELENZA should be advised to use their bronchodilators before taking RELENZA.
Neuropsychiatric Events
Inform patients with influenza (the flu), particularly children and adolescents, they may be at an increased risk of seizures, confusion, or abnormal behavior early in their illness. These events may occur after beginning RELENZA or may occur when flu is not treated. These events are uncommon but may result in accidental injury to the patient. Therefore, patients should be observed for signs of unusual behavior and a healthcare professional should be contacted immediately if the patient shows any signs of unusual behavior [see Warnings and Precautions (5.3)].
Risk of Influenza Transmission to Others
Inform patients that the use of RELENZA for treatment of influenza has not been shown to reduce the risk of transmission of influenza to others.
Missed Dose
Instruct patients that if they miss a dose of RELENZA, to take it as soon as they remember. If it is 2 hours or less before their next dose, instruct patients not to take the missed dose and to take the next dose of RELENZA at the next scheduled time. Advise patients not to double their next dose or take more than the prescribed dose.
Instructions for Use
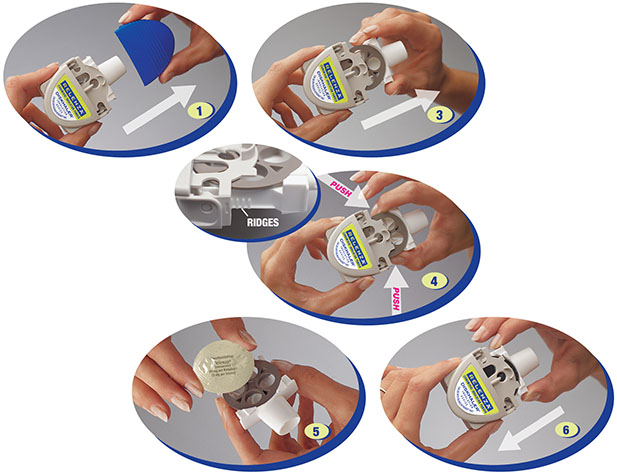
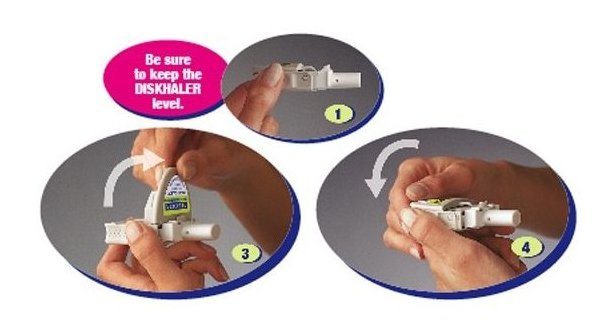
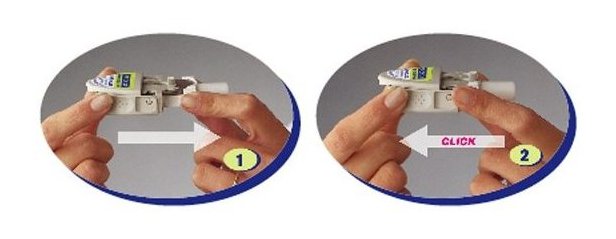
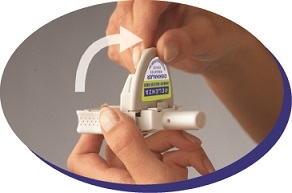
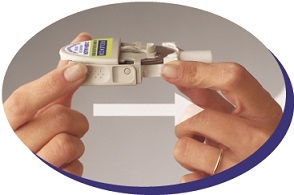
Instruct patients in use of the delivery system. Instructions should include a demonstration whenever possible. For the proper use of RELENZA, the patient should read and follow carefully the accompanying Instructions for Use.
If RELENZA is prescribed for children, it should be used only under adult supervision and instruction, and the supervising adult should first be instructed by a healthcare professional [see Dosage and Administration (2.1)].
Trademarks are owned by or licensed to the GSK group of companies.
GlaxoSmithKline
Research Triangle Park, NC 27709
©2018 GSK group of companies or its licensor.
RLZ: 12PI
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
RELENZA (ruh-LENS-uh)
(zanamivir inhalation powder)
for oral inhalation use
What is RELENZA?
RELENZA is a prescription medicine used to:
- treat the flu (influenza A and B virus) in people who are aged 7 years and older who have had flu symptoms for no more than 2 days.
- help prevent the flu in people who are aged 5 years and older.
RELENZA is not recommended for the treatment or prevention of the flu in people with breathing problems, such as asthma and chronic obstructive pulmonary disease (COPD).
RELENZA does not treat or prevent illness that is caused by infections other than the influenza virus (A and B).
Using RELENZA for the treatment of the flu has not been shown to reduce the risk of spreading the flu to others.
It is not known if RELENZA is:
- effective for the treatment of flu in people with breathing problems.
- effective for the prevention of flu in people who live in nursing homes.
- safe and effective for the treatment of flu in children younger than 7 years.
- safe and effective for the prevention of flu in children younger than 5 years.
RELENZA does not take the place of receiving a flu vaccination. Talk to your healthcare provider about when you should receive an annual flu vaccination.
Do not take RELENZA if you are allergic to zanamivir or any other ingredient of RELENZA, including milk proteins. See the end of this Patient Information leaflet for a complete list of ingredients in RELENZA.
Before taking RELENZA, tell your healthcare provider about all of your medical conditions, including if you:
- have any breathing problems, such as asthma or COPD.
- received live attenuated influenza vaccine (LAIV) in your nose (intranasal) in the past 2 weeks.
- are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if RELENZA passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I take RELENZA?
Read the Instructions for Use at the end of this Patient Information for detailed information on how to take RELENZA.
- Take RELENZA exactly as your healthcare provider tells you to.
- RELENZA is taken by oral inhalation using a DISKHALER.
- If you are scheduled to use an inhaled bronchodilator at the same time as RELENZA, use the inhaled bronchodilator before taking RELENZA.
- Each dose of RELENZA requires 2 inhalations. Each inhalation requires 1 blister.
For the treatment of flu in people aged 7 years and older, take RELENZA as follows:
- Take each dose of RELENZA as 2 inhalations (use 1 blister per inhalation) 2 times a day for 5 days.
- On the first day, wait at least 2 hours between each dose.
- Starting on the second day, wait at least 12 hours between each dose.
To help prevent the flu in people aged 5 years and older, take RELENZA as follows:
- Take each dose of RELENZA as 2 inhalations (use 1 blister per inhalation) 1 time each day for 10 or 28 days as prescribed by your healthcare provider.
- Take RELENZA at the same time each day.
If you miss a dose of RELENZA, take it as soon as you remember. If it is 2 hours or less before your next dose, do not take the missed dose. Take your next dose of RELENZA at your scheduled time. Do not take 2 doses at the same time.
What are the possible side effects of RELENZA?
RELENZA can cause serious side effects, including:
- Bronchospasm (wheezing). Serious breathing problems, including death, have happened during treatment with RELENZA in people with and without a history of breathing problems. 41TStop taking RELENZA and get emergency medical help right away if you develop:
- ᴏ worsening breathing
- ᴏ shortness of breath
- ᴏ chest pain or tightness
- ᴏ cough
- ᴏ If you have breathing problems, such as asthma and COPD and your healthcare provider has prescribed RELENZA, you should have a fast‑acting, inhaled bronchodilator available for your use. See “How should I take RELENZA?”
- Serious allergic reactions. RELENZA can cause serious allergic reactions. Stop taking RELENZA and get emergency medical help right away if you get any of the following symptoms:
- ᴏ skin rash or hives
- ᴏ your skin blisters and peels
- ᴏ blisters or sores in your mouth
- ᴏ itching
- ᴏ swelling of your face, eyes, lips, tongue, or throat
- ᴏ trouble breathing
- ᴏ chest pain or tightness
- Change in behavior. People, especially children, who have the flu, can develop nervous system problems and abnormal behavior that can lead to death. During treatment with RELENZA, tell your healthcare provider right away if you or your child have confusion, speech problems, shaky movements, seizures, or start hearing voices or seeing things that are not really there (hallucinations).
The most common side effects of RELENZA include:
- ᴏ headaches
- ᴏ diarrhea
- ᴏ nausea
- ᴏ vomiting
- ᴏ irritation of the nose
- ᴏ airway irritation (bronchitis)
- ᴏ cough
- ᴏ sinusitis
- ᴏ ear, nose, and throat infections
- ᴏ dizziness
Other kinds of infections can appear like flu or happen along with flu, and may need different kinds of treatment. Call your healthcare provider if you feel worse or develop new symptoms during or after treatment with RELENZA, or if your flu symptoms do not start to get better.
These are not all of the possible side effects of RELENZA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1‑800‑FDA‑1088.
How should I store RELENZA?
- Store RELENZA at room temperature below 77°F (25°C).
- Do not puncture any RELENZA ROTADISK blister until you are ready to take your dose using the DISKHALER.
Keep RELENZA and all medicines out of the reach of children.
General information about the safe and effective use of RELENZA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use RELENZA for a condition for which it was not prescribed. Do not give RELENZA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about RELENZA that is written for health professionals.
What are the ingredients in RELENZA?
Active ingredient: zanamivir
Inactive ingredients: lactose (which contains milk proteins)
GlaxoSmithKline
Research Triangle Park, NC 27709
RELENZA, ROTADISK, and DISKHALER are trademarks owned by or licensed to the GSK group of companies.
©2018 the GSK group of companies or its licensor.
RLZ:8PIL
For more information call -1-888-825-5249.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 06/2018
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
-
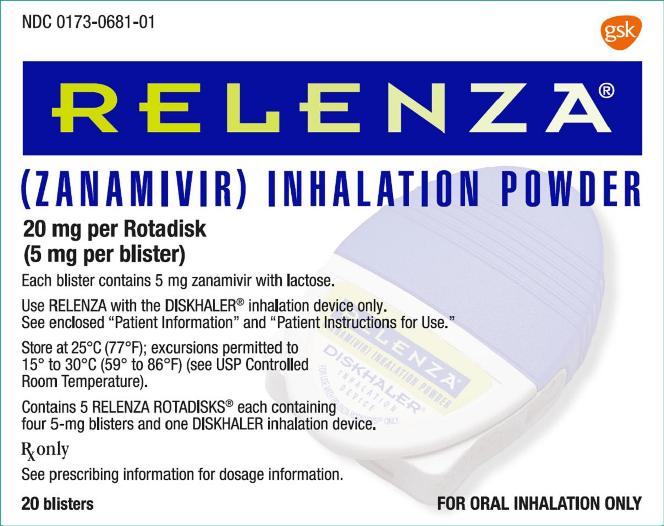
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC: 0173-0681-01
RELENZA®
(ZANAMIVIR) INHALATION POWDER
20 mg per Rotadisk
(5 mg per blister)
Each blister contains 5 mg zanamivir with lactose.
Use RELENZA with DISKHALER® inhalation device only.
See enclosed “Patient Information” and “Patient Instructions for Use.”
Store at 25oC (77oF); excursions permitted to 15o to 30oC (59o to 86oF) (see USP Controlled Room Temperature).
Contains 5 RELENZA ROTADISKS® each containing four 5-mg blisters and one DISKHALER inhalation device.
Rx only
See prescribing information for dosage information.
20 blisters
FOR ORAL INHALATION ONLY
Made in UK
Rev. 2/17
62000000011902
-
INGREDIENTS AND APPEARANCE
RELENZA
zanamivir powderProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0173-0681 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZANAMIVIR (UNII: L6O3XI777I) (ZANAMIVIR - UNII:L6O3XI777I) ZANAMIVIR 5 mg Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0173-0681-01 5 in 1 CARTON 09/22/1999 1 4 in 1 PACKAGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021036 09/22/1999 Labeler - GlaxoSmithKline LLC (167380711)
Trademark Results [RELENZA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 RELENZA 78452022 3049319 Live/Registered |
Glaxo Group Limited 2004-07-16 |
 RELENZA 75975285 2101718 Dead/Cancelled |
Glaxo Group Limited 1994-06-21 |
 RELENZA 74540557 not registered Dead/Abandoned |
Glaxo Group Limited 1994-06-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.