WAINUA- eplontersen injection, solution
WAINUA by
Drug Labeling and Warnings
WAINUA by is a Prescription medication manufactured, distributed, or labeled by AstraZeneca Pharmaceuticals LP, AstraZeneca PLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use WAINUA safely and effectively. See full prescribing information for WAINUA.
WAINUA® (eplontersen) injection, for subcutaneous use
Initial U.S. Approval: 2023INDICATIONS AND USAGE
WAINUA is a transthyretin-directed antisense oligonucleotide indicated for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis in adults. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Injection: 45 mg/0.8 mL in a single-dose autoinjector. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
Reduced Serum Vitamin A Levels and Recommended Supplementation: Supplement with the recommended daily allowance of vitamin A. Refer to an ophthalmologist if ocular symptoms suggestive of vitamin A deficiency occur. (5.1)
ADVERSE REACTIONS
Most common adverse reactions (that occurred in at least 9% of patients treated with WAINUA) were vitamin A decreased and vomiting. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AstraZeneca at 1-800-236-9933 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Reduced Serum Vitamin A Levels and Recommended Supplementation
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosage of WAINUA is 45 mg administered by subcutaneous injection once monthly [see Dosage and Administration (2.2)].
Missed Dose
Administer WAINUA as soon as possible after a missed dose. Resume dosing at monthly intervals from the date of the most recently administered dose.
2.2 Administration Instructions
Prior to initiation, train patients and/or caregivers on proper preparation and administration of WAINUA [see Instructions for Use].
Remove the single-dose autoinjector from the refrigerator 30 minutes prior to the injection and allow to warm to room temperature. Do not use other warming methods.
Inspect WAINUA visually for particulate matter and discoloration prior to administration. The solution should appear colorless to yellow. Do not use if cloudiness, particulate matter, or discoloration is observed prior to administration.
Administer WAINUA as a subcutaneous injection into the abdomen or upper thigh region. The back of the upper arm can also be used as an injection site if a healthcare provider or caregiver administers the injection.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Reduced Serum Vitamin A Levels and Recommended Supplementation
WAINUA treatment leads to a decrease in serum vitamin A levels [see Adverse Reactions (6.1), Use in Specific Populations (8.1), and Clinical Pharmacology (12.2)].
Supplementation at the recommended daily allowance of vitamin A is advised for patients taking WAINUA. Higher doses than the recommended daily allowance of vitamin A should not be given to try to achieve normal serum vitamin A levels during treatment with WAINUA, as serum vitamin A levels do not reflect the total vitamin A in the body.
Patients should be referred to an ophthalmologist if they develop ocular symptoms suggestive of vitamin A deficiency (e.g., night blindness, dry eyes).
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling:
- Reduced Serum Vitamin A Levels and Recommended Supplementation [see Warnings and Precautions (5.1)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of WAINUA cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In Study 1 [see Clinical Studies (14)], a total of 144 patients with polyneuropathy caused by hereditary transthyretin-mediated amyloidosis (hATTR amyloidosis) were randomized to WAINUA and received at least one dose of WAINUA. Of these, 141 patients received at least 6 months of treatment and 107 patients received at least 12 months of treatment. The mean duration of treatment was 15 months (range: 1.9 to 19.4 months). The median patient age at baseline was 52 years and 69% of the patients were male. Seventy-eight percent of patients treated with WAINUA were White, 15% were Asian, 4% were Black, 2% were reported as other races, and <1% were multiple races. Fifty-nine percent of patients had the Val30Met variant in the transthyretin gene; the remaining patients had one of 19 other variants. At baseline, 80% of patients were in Stage 1 of the disease and 20% were in Stage 2 with a mean duration from polyneuropathy diagnosis of 47 months. The mean duration from onset of polyneuropathy symptoms was 68 months.
Table 1 lists the adverse reactions that occurred in at least 5% of patients treated with WAINUA in Study 1.
Table 1: Adverse Reactions Reported in at least 5% of Patients Treated with WAINUA (Study 1) - * Vitamin A decreased includes vitamin A deficiency and vitamin A decrease.
- † Injection site reactions includes erythema, pain, and pruritis.
Adverse Reaction
WAINUA
N=144
%
Vitamin A decreased*
15
Vomiting
9
Proteinuria
8
Injection site reactions†
7
Blurred vision
6
Cataract
6
Three serious adverse reactions of atrioventricular (AV) heart block (2%) occurred in WAINUA-treated patients, including 1 case of complete AV block.
Laboratory Tests
Vitamin A Decrease
In Study 1, patients were instructed to take the recommended daily allowance of vitamin A [see Warnings and Precautions (5.1)]. All patients treated with WAINUA had normal vitamin A levels at baseline, 95% of patients developed low vitamin A levels during the study. In some cases, the decreased vitamin A level was reported as an adverse reaction.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on WAINUA use in pregnant women to inform drug-associated risk of adverse developmental outcomes. WAINUA treatment leads to a decrease in serum vitamin A levels, and vitamin A supplementation is advised for patients taking WAINUA. Vitamin A is essential for normal embryofetal development; however, excessive levels of vitamin A are associated with adverse developmental effects. The effect of vitamin A supplementation on the fetus in the setting of a reduction in maternal serum TTR caused by WAINUA administration is unknown [see Clinical Pharmacology (12.2) and Warnings and Precautions (5.1)].
No adverse developmental effects were observed when eplontersen or a mouse-specific surrogate was administered to mice prior to mating and continuing throughout organogenesis [see Animal Data].
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Data
Animal Data
Subcutaneous administration of eplontersen (0, 5, 25, or 75 mg/kg) or a mouse-specific surrogate (25 mg/kg) to male and female mice weekly prior to and during mating and administration continued every other day in females throughout the period of organogenesis resulted in no adverse effects on embryofetal development.
8.2 Lactation
Risk Summary
There is no information regarding the presence of eplontersen in human milk, the effects on the breast-fed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for WAINUA and any potential adverse effects on the breast-fed infant from WAINUA or from the underlying maternal condition.
8.5 Geriatric Use
No dose adjustment is required in patients ≥65 years of age [see Clinical Pharmacology (12.3)]. In Study 1 [see Clinical Studies (14)], 44 (31%) patients were 65 to 74 years of age, and 8 (5.6%) patients were ≥75 years of age. No overall differences in safety or effectiveness were observed between these patients and younger adult patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
No dose adjustment is necessary in patients with mild to moderate renal impairment (estimated glomerular filtration rate [eGFR] ≥30 to <90 mL/min/1.73 m2) [see Clinical Pharmacology (12.3)].
WAINUA has not been studied in patients with severe renal impairment or end-stage renal disease.
8.7 Hepatic Impairment
No dose adjustment is necessary in patients with mild hepatic impairment (total bilirubin ≤1 x ULN and AST >1 x ULN, or total bilirubin >1.0 to 1.5 x ULN and any AST) [see Clinical Pharmacology (12.3)].
WAINUA has not been studied in patients with moderate or severe hepatic impairment.
-
11 DESCRIPTION
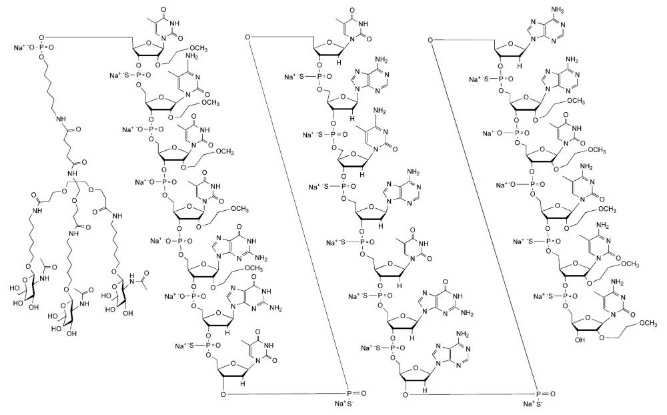
Eplontersen is a transthyretin-directed antisense oligonucleotide (ASO), covalently linked to a ligand containing three N-acetyl galactosamine (GalNAc) residues to enable delivery of the ASO to hepatocytes.
WAINUA contains eplontersen sodium as the active ingredient. Eplontersen sodium is a white to yellow solid and it is freely soluble in water and in phosphate buffer. The molecular formula of eplontersen sodium is C296H417N77O156P20S13Na20 and the molecular weight is 9046.1 daltons. The chemical name of eplontersen sodium is DNA, d([2′-O-(2-methoxyethyl)]m5rU-sp-[2′-O-(2-methoxyethyl)]m5rC-[2′-O-(2-methoxyethyl)]m5rU-[2′-O-(2-methoxyethyl)]m5rU-[2′-O-(2-methoxyethyl)]rG-G-sp-T-sp-T-sp-A-sp-m5C-sp-A-sp-T-sp-G-sp-A-sp-A-sp-[2′-O-(2-methoxyethyl)]rA-[2′-O-(2-methoxyethyl)]m5rU-[2′-O-(2-methoxyethyl)]m5rC-sp-[2′-O-(2-methoxyethyl)]m5rC-sp-[2′-O-(2-methoxyethyl)]m5rC), 5′-[26-[[2-(acetylamino)-2-deoxy-β-D-galactopyranosyl]oxy]-14,14-bis[[3-[[6-[[2-(acetylamino)-2-deoxy-β-D-galactopyranosyl]oxy]hexyl]amino]-3-oxopropoxy]methyl]-8,12,19-trioxo-16-oxa-7,13,20-triazahexacos-1-yl hydrogen phosphate], sodium salt (1:20).
The structure of eplontersen sodium is presented below:
WAINUA is a sterile, preservative-free, aqueous solution for subcutaneous injection. Each single-dose autoinjector contains 45 mg eplontersen (equivalent to 47 mg eplontersen sodium) in 0.8 mL of solution. The solution also contains 0.868 mg dibasic sodium phosphate, anhydrous (buffering agent); 0.238 mg monobasic sodium phosphate, dihydrate (buffering agent); 4.2 mg sodium chloride (tonicity modifier); water for injection; and may include hydrochloric acid and/or sodium hydroxide for pH adjustment between 6.9 - 7.9. Each dose of WAINUA injection contains less than 5 mg of sodium and less than 5 mg of phosphorus.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Eplontersen is an antisense oligonucleotide-GalNAc conjugate that causes degradation of mutant and wild-type TTR mRNA through binding to the TTR mRNA, which results in a reduction of serum TTR protein and TTR protein deposits in tissues.
12.2 Pharmacodynamics
In Study 1 [see Clinical Studies (14)], following administration of the recommended WAINUA dosage every 4 weeks to patients with hATTR amyloidosis, a decrease in serum TTR levels was observed at the first assessment and the (least square) mean serum TTR at Week 35 was reduced by 81% from baseline. Similar TTR reductions were observed across subgroups including Val30Met variant status, body weight, sex, age, or race.
Eplontersen also reduced the mean steady state serum vitamin A by 71% by Week 37 [see Warnings and Precautions (5.1)].
12.3 Pharmacokinetics
The pharmacokinetic (PK) properties of WAINUA were evaluated following subcutaneous administration of single and multiple doses (once every 4 weeks) in healthy subjects and multiple doses (once every 4 weeks) in patients with hATTR amyloidosis.
Eplontersen Cmax and AUC showed a slightly greater than dose-proportional increase following single subcutaneous doses ranging from 45 to 120 mg (i.e., 1- to 2.7-times the recommended dose) in healthy volunteers.
Population estimates (mean ± SD) of steady state maximum concentrations (Cmax), and area under the curve (AUCτ) were 283 ± 152 ng/mL, and 2190 ± 689 ng/mL, respectively, following 45 mg monthly dosing in patients with hATTR amyloidosis. No accumulation of eplontersen Cmax and AUC was observed in repeated dosing (once every 4 weeks).
Absorption
Following subcutaneous administration, eplontersen is absorbed with the time to maximum plasma concentrations of approximately 2 hours, based on population estimates.
Distribution
Eplontersen is expected to distribute primarily to the liver and kidney cortex after subcutaneous dosing. Eplontersen is bound to human plasma proteins (>98%) in vitro. The population estimate for the apparent central volume of distribution is 12 L and the apparent peripheral volume of distribution is 11,100 L.
Elimination
The terminal elimination half-life is approximately 3 weeks.
Specific Populations
Population pharmacokinetic and pharmacodynamic analysis showed no clinically meaningful differences in the pharmacokinetics or pharmacodynamics of eplontersen based on age, body weight, sex, race, Val30Met variant status, mild and moderate renal impairment (eGFR≥30 to <90 mL/min), or mild hepatic impairment (total bilirubin ≤1 x ULN and AST > 1 x ULN, or total bilirubin >1.0 to 1.5 x ULN and any AST). Eplontersen has not been studied in patients with severe renal impairment, end-stage renal disease, or in patients with moderate to severe hepatic impairment, or in patients with prior liver transplant.
Drug Interaction Studies
No clinical drug-drug interaction studies have been performed with eplontersen. In vitro studies show that eplontersen is not a substrate or inhibitor of transporters, does not interact with highly plasma protein bound drugs, and is not an inhibitor or inducer of cytochrome P450 (CYP) enzymes. Oligonucleotide therapeutics, including eplontersen, are not typically substrates of CYP enzymes. Therefore, eplontersen is not expected to cause or be affected by drug-drug interactions mediated through drug transporters, plasma protein binding or CYP enzymes.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies (ADAs) is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of ADAs in the study described below with the incidence of anti-drug antibodies in other studies, including those of eplontersen.
In Study 1, with duration of treatment up to 85 weeks (mean treatment duration of 445 days (63.2 weeks), range: 57 to 582 days), 53 out of 144 (37%) patients developed treatment-emergent ADAs during treatment with WAINUA. The presence of ADAs did not affect eplontersen plasma Cmax or AUC, but increased Ctrough. Although anti-drug antibody development was not found to affect the pharmacokinetics, pharmacodynamics, safety, or efficacy of WAINUA in these patients, the available data are too limited to make definitive conclusions.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a 26-week carcinogenicity study in male and female Tg.rasH2 mice, subcutaneous administration of eplontersen (0, 250, 500, or 1500 mg/kg) monthly for 26 weeks resulted in no increase in neoplasms.
Mutagenesis
Eplontersen was negative for genotoxicity in in vitro (bacterial reverse mutation, chromosomal aberration in Chinese hamster lung cells) and in vivo (mouse bone marrow micronucleus) assays.
Impairment of Fertility
Subcutaneous administration of eplontersen (0, 5, 25, or 75 mg/kg) or a mouse-specific surrogate (25 mg/kg) to male and female mice weekly prior to and during mating and continuing in females throughout the period of organogenesis resulted in no adverse effects on fertility.
-
14 CLINICAL STUDIES
The efficacy of WAINUA was demonstrated in a randomized, open-label, multicenter clinical trial in adult patients with polyneuropathy caused by hATTR amyloidosis (Study 1; NCT04136184). Patients were randomized in a 6:1 ratio to receive either 45 mg of WAINUA once every 4 weeks (N=144), or 284 mg of inotersen once per week (N=24), respectively, as subcutaneous injections. Ninety-seven percent of WAINUA-treated patients and 83% of inotersen-treated patients completed at least 35 weeks of the assigned treatment.
Efficacy assessments were based on a comparison of the WAINUA arm of Study 1 with an external placebo group (N=60) in another study (NCT01737398) composed of a comparable population of adult patients with polyneuropathy caused by hATTR amyloidosis.
The efficacy endpoints were the change from baseline to Week 35 in the modified Neuropathy Impairment Scale+7 (mNIS+7) composite score and the change from baseline to Week 35 in the Norfolk Quality of Life-Diabetic Neuropathy (QoL-DN) total score.
The mNIS+7 is an objective assessment of neuropathy and comprises the neuropathy impairment score (NIS) and Modified +7 composite scores. In the version of the mNIS+7 used in the trial, the NIS objectively measures deficits in cranial nerve function, muscle strength, reflexes, and sensations, and the Modified +7 assesses heart rate response to deep breathing, quantitative sensory testing (touch-pressure and heat-pain), and peripheral nerve electrophysiology. The validated version of the mNIS+7 score used in the trial has a range of -22.3 to 346.3 points, with higher scores representing a greater severity of disease.
The clinical meaningfulness of effects on the mNIS+7 was assessed by the change from baseline to Week 35 in Norfolk Quality of Life-Diabetic Neuropathy (QoL-DN) total score. The Norfolk QoL-DN scale is a patient-reported assessment that evaluates the subjective experience of neuropathy in the following domains: physical functioning/large fiber neuropathy, activities of daily living, symptoms, small fiber neuropathy, and autonomic neuropathy. The version of the Norfolk QoL-DN that was used in the trial has a range from -4 to 136 points, with higher scores representing greater impairment.
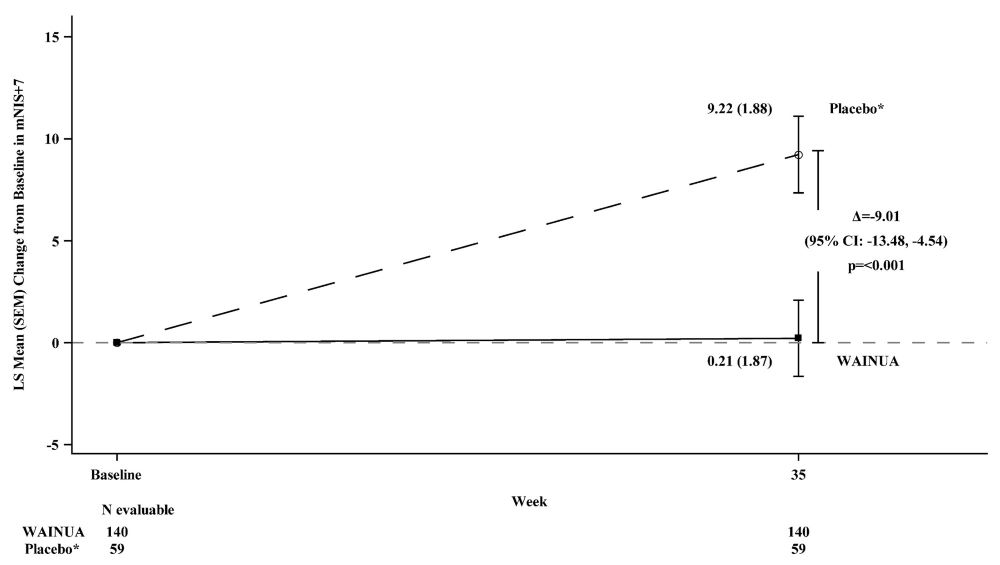
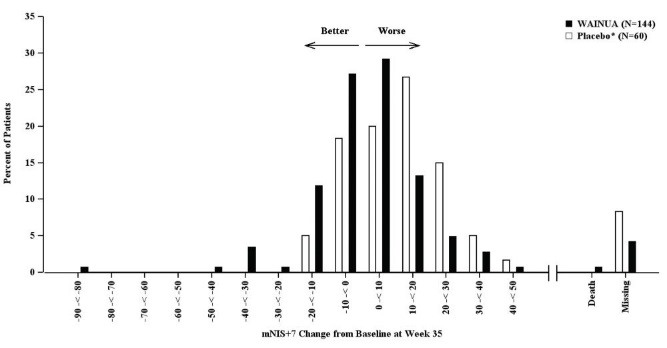
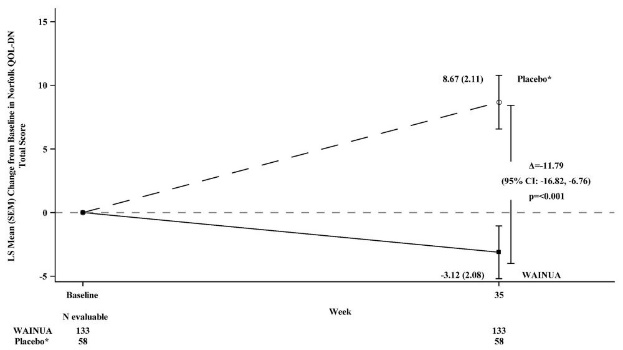
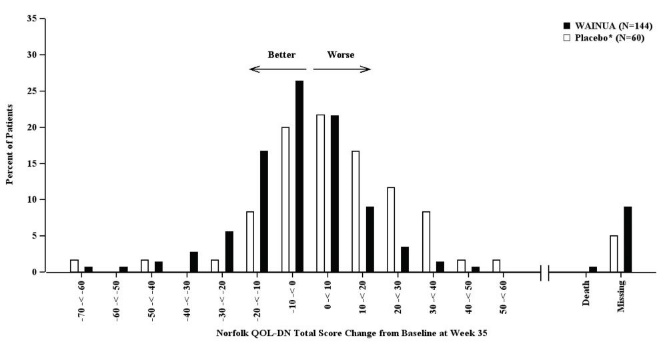
Treatment with WAINUA resulted in statistically significant improvements in the mNIS+7 and the Norfolk QoL-DN total scores, compared to the external placebo control (p<0.001) at Week 35 (Table 2, Figures 1 and 3). The distributions of changes in mNIS+7 and Norfolk QoL-DN scores from baseline to Week 35 by percent of patients in each category are shown in Figure 2 and Figure 4, respectively.
Table 2: Clinical Efficacy Results (Comparison of WAINUA Treatment in Study 1 to an External Placebo Control*) - * External placebo group from another randomized controlled trial (NCT01737398).
- † Based on an analysis of covariance (ANCOVA) model. Patients with a missing mNIS+7 or Norfolk QoL-DN at Week 35 had values multiply imputed using an imputation model.
Endpoint
Baseline, Mean (SD)
Change from Baseline to
Week 35,
LS Mean (SEM)Treatment Difference
LS Mean(95% CI)
p-value
WAINUA
N = 140(Study 1)
Placebo*
N = 59 (NCT01737398)
WAINUA
(Study 1)
Placebo* (NCT01737398)
WAINUA- Placebo*
mNIS+7†
79.6
(42.3)
74.1 (39.0)
0.2 (1.9)
9.2 (1.9)
-9.0
(-13.5, -4.5)<0.001
Norfolk QOL-DN†
43.5
(26.3)
48.6 (27.0)
-3.1 (2.1)
8.7 (2.1)
-11.8
(-16.8, -6.8)<0.001
CI = confidence interval; LS mean = least squares mean; mNIS = modified Neuropathy Impairment Score; QoL-DN = Quality of Life-Diabetic Neuropathy; SD = standard deviation; SEM = standard error of the mean.
Figure 1: Change from Baseline in mNIS+7 at Week 35 (Comparison of WAINUA Treatment in Study 1 to an External Placebo Control*)
* External placebo group from another randomized controlled trial (NCT01737398).
Based on an analysis of covariance (ANCOVA) model. Patients with a missing mNIS+7 at Week 35 had values multiply imputed using an imputation model.Figure 2: Histogram of mNIS+7 Change from Baseline at Week 35 (Comparison of WAINUA Treatment in Study 1 to an External Placebo Control*)
* External placebo group from another randomized controlled trial (NCT01737398).
Categories are mutually exclusive; patients who died before Week 35 are summarized in the Death category only; missing mNIS+7 values not imputed; patients with missing values at Week 35 presented in the Missing category.Figure 3: Change from Baseline in Norfolk QoL-DN Total Score at Week 35 (Comparison of WAINUA Treatment in Study 1 to an External Placebo Control*)
* External placebo group from another randomized controlled trial (NCT01737398).
Based on an analysis of covariance (ANCOVA) model. Patients with a missing Norfolk QoL-DN at Week 35 had values multiply imputed using an imputation model.Figure 4: Histogram of Norfolk QoL-DN Total Score Change from Baseline at Week 35 (Comparison of WAINUA Treatment in Study 1 to an External Placebo Control*)
* External placebo group from another randomized controlled trial (NCT01737398).
Categories are mutually exclusive; patients who died before Week 35 are summarized in the Death category only; missing Norfolk QoL-DN values not imputed; patients with missing values at Week 35 presented in the Missing category.Patients receiving WAINUA experienced similar improvements relative to those in the external placebo in mNIS+7, and Norfolk QoL-DN score across subgroups including age, sex, race, region, Val30Met variant status, and disease stage.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
WAINUA injection is a sterile, preservative-free, clear, colorless to yellow solution supplied in a single-dose autoinjector. Each autoinjector of WAINUA is filled to deliver 0.8 mL of solution containing 45 mg of eplontersen.
WAINUA is available in cartons containing one 45 mg single-dose autoinjector each.
The NDC is: 0310-9400-01.
16.2 Storage and Handling
Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton protected from light.
If needed, WAINUA can be kept at room temperature (up to 30°C [86°F]) in the original carton for up to 6 weeks. If not used within the 6 weeks stored at room temperature, discard WAINUA.
Do not freeze. Do not expose to heat.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Recommended Vitamin A Supplementation
Inform patients that WAINUA treatment leads to a decrease in vitamin A levels measured in the serum. Instruct patients to take the recommended daily allowance of vitamin A. Advise patients to contact their healthcare provider if they experience ocular symptoms suggestive of vitamin A deficiency (e.g., night blindness, dry eyes) and refer them to an ophthalmologist if they develop these symptoms [see Warnings and Precautions (5.1)].
Pregnancy
Instruct patients that if they are pregnant or plan to become pregnant while taking WAINUA they should inform their healthcare provider. Advise patients of the potential risk to the fetus, including that WAINUA treatment leads to a decrease in serum vitamin A levels [see Use in Special Populations (8.1)].
Distributed by: AstraZeneca Pharmaceuticals LP, Wilmington, DE 19850
©AstraZeneca 2025
-
PATIENT INFORMATION
PATIENT INFORMATION
WAINUA [way-noo’-ah]
(eplontersen)
injection, for subcutaneous use
What is WAINUA?
WAINUA is a prescription medicine used to treat adults with polyneuropathy of hereditary transthyretin-mediated amyloidosis.
It is not known if WAINUA is safe and effective in children.
Before you take WAINUA, tell your healthcare provider if you:
- are pregnant or plan to become pregnant. It is not known if WAINUA can harm your unborn baby. Changes in vitamin A levels and vitamin A supplementation related to use of WAINUA may harm your unborn baby. Tell your healthcare provider if you become pregnant or think you may be pregnant while taking WAINUA.
- are breastfeeding or plan to breastfeed. It is not known if WAINUA can pass into your breast milk or harm your baby. Talk with your healthcare provider about the best way to feed your baby while you are using WAINUA.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Especially tell your healthcare provider if you take:
- Vitamin A or beta-carotene supplements.
Ask your healthcare provider or pharmacist if you are not sure if you take any of these medicines. Know the medicines you take. Keep a list of them to show your healthcare provider or pharmacist when you get a new medicine.
How should I take WAINUA?
- Read the detailed Instructions for Use that come with your WAINUA single-dose autoinjector.
- Your healthcare provider will show you or your caregiver how to inject WAINUA the first time.
- If you or your caregiver have any questions, ask your healthcare provider.
- WAINUA is injected under your skin (subcutaneously) in your stomach area (abdomen), or the front of your upper legs (thighs) by you or a caregiver. A caregiver may also give you an injection of WAINUA in the outer area of your upper arm.
- Follow your healthcare provider’s instructions on when to inject WAINUA.
- WAINUA should be injected 1 time on the same day of each month.
- If you miss a dose, take the missed dose as soon as possible. Then inject WAINUA 1 month from the date of your last dose to get back on a monthly dosing schedule. If you have questions about your schedule, ask your healthcare provider.
What are the possible side effects of WAINUA?
WAINUA may cause side effects, including:
- Low vitamin A level. Low vitamin A level is a serious, but common side effect of treatment with WAINUA. Your healthcare provider should tell you to take vitamin A supplements while using WAINUA. Do not take more than the amount of vitamin A your healthcare provider has recommended. Call your healthcare provider if you develop eye problems such as difficulty seeing at night or in low lit areas (night blindness), or dry eyes. If you develop eye problems while receiving treatment with WAINUA, your healthcare provider should send you to see an eye doctor (ophthalmologist).
The most common side effects of WAINUA include decreased vitamin A and vomiting.
These are not all of the possible side effects of WAINUA. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1‑800-FDA-1088.
How should I store WAINUA?
- Store WAINUA in the refrigerator between 36°F to 46°F (2°C to 8°C) in the original carton.
- WAINUA can also be kept at room temperature that is no higher than 86°F (30°C) in the original carton for up to 6 weeks.
- Do not let WAINUA reach temperatures above 86°F (30°C).
- If you do not use WAINUA kept at room temperature within 6 weeks, throw it away.
- Do not freeze.
- Do not expose WAINUA to heat.
- Protect from light.
Keep WAINUA and all medicines out of the reach of children.
General information about the safe and effective use of WAINUA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. Do not use WAINUA for a condition for which it was not prescribed. Do not give WAINUA to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about WAINUA that is written for health professionals.
What are the ingredients in WAINUA?
Active ingredient: eplontersen sodium.
Inactive ingredients: dibasic sodium phosphate, anhydrous; monobasic sodium phosphate, dihydrate; sodium chloride; water for injection, and may include hydrochloric acid and sodium hydroxide for pH adjustment.
Distributed by: AstraZeneca Pharmaceuticals LP, Wilmington, DE 19850©AstraZeneca 2023
For more information, go to https://www.wainua.com or call 1-800-236-9933. If you still have questions, contact your healthcare provider.
This Patient Information has been approved by the U.S. Food and Drug Administration Approved: 12/2023
-
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
WAINUA [way-noo’-ah]
(eplontersen)
injection, for subcutaneous use
Single-dose autoinjector45 mg/0.8 mL
This Instructions for Use contains information on how to inject WAINUA using the autoinjector.
Read this Instructions for Use before you start using the WAINUA autoinjector and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition and your treatment.
Your healthcare provider should show you or your caregiver how to use the autoinjector the right way. If you or your caregiver have any questions, talk to your healthcare provider.
- Important information you need to know before using WAINUA
- Keep your autoinjector and all medicines out of the sight and reach of children.
- WAINUA is for use by injection under the skin (subcutaneous use) only.
- Each WAINUA autoinjector contains 1 dose and can only be used 1 time.
- Do not share your autoinjector with anyone.
-
Do not use the autoinjector if it has:
- ∘ been frozen
- ∘ been dropped, damaged, or appears to be tampered with
- ∘ passed the expiration date (EXP)
- Storing the WAINUA autoinjector
- Store the WAINUA autoinjector in the refrigerator between 36°F to 46°F (2°C to 8°C) in the original carton to protect from light. Do not freeze.
- If needed, the WAINUA autoinjector may be stored at room temperature up to 86°F (30°C) for up to a maximum of 6 weeks protected from light.
- If you do not use the WAINUA autoinjector within 6 weeks at room temperature, throw it away in an FDA-cleared sharps disposal container.
-
Keep the autoinjector in the carton until ready to use.
Keep your autoinjector and all medicines out of the sight and reach of children.
Overview of your WAINUA autoinjector
Do not remove the cap until just before you give the injection.
Do not touch the orange needle guard.
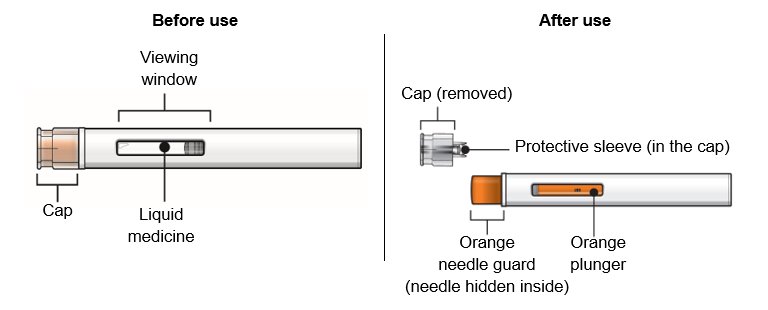
How does the WAINUA autoinjector work?
Make sure you read the entire instructions before uncapping and injecting.
The injection starts automatically when the orange needle guard is pushed against the skin.
The autoinjector must be held firmly against the skin to allow it to deliver the full dose of the medicine.
The injection is complete only when the orange plunger rod fills the viewing window (See “Overview of your WAINUA autoinjector”, After use).
Preparing to inject WAINUA using the autoinjector
Step 1 – Gather supplies for your injection
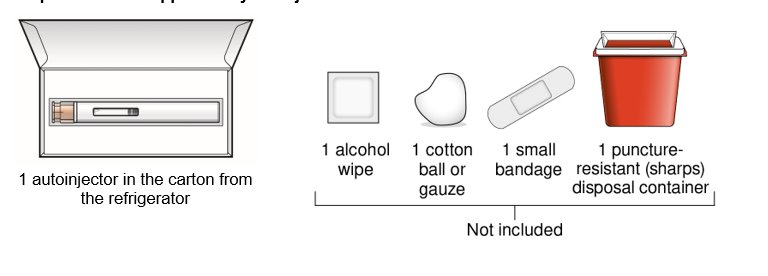
Step 2 – Wait 30 minutes
Keep the autoinjector in the original carton after removing it from the refrigerator and allow to come to room temperature for 30 minutes before injecting.
- Do not warm up in any other way. For example, do not warm it in a microwave or hot water, or near other heat sources.
- Keep it away from light or direct sunlight.

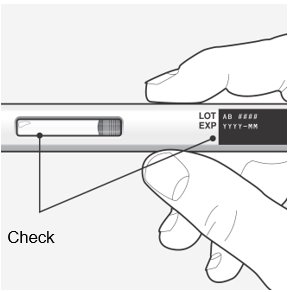
Step 3 – Remove from the carton and check the autoinjector and medicine
Check the autoinjector for damage.
- Do not use if damaged.
Check the expiration (EXP) date.
- Do not use if the expiration date has passed.
Check the liquid through the viewing window.
- It is normal to see small air bubbles in the liquid.
- The liquid should be clear and colorless to slightly yellow.
- Do not use if the liquid is cloudy, discolored, or contains large particles.
Injecting with your autoinjector
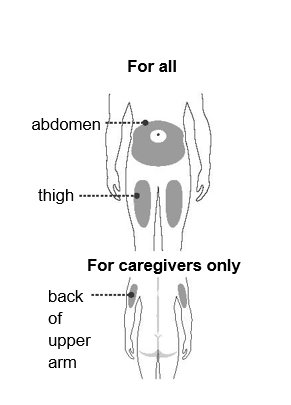
Step 4 – Choose an injection site
You or your caregiver can inject in the front of your thigh or your stomach (abdomen).
A caregiver may inject you in the back of your upper arm.
Do not try to inject yourself in the back of your upper arm.
For each injection, choose an injection site that is at least 1 inch (3 cm) away from where you last injected.
Do not inject:
- into the 2-inch (5-cm) area around your belly button
- where the skin is red, warm, tender, bruised, scaly, or hard
- into scars, damaged, discolored, or tattooed skin
- through clothing
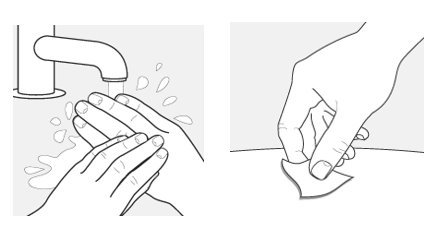
Step 5 – Wash your hands and clean the injection site
Wash your hands well with soap and water.
Clean the injection site with an alcohol wipe or with soap and water. Let the site air dry.
- Do not touch the cleaned area before injecting.
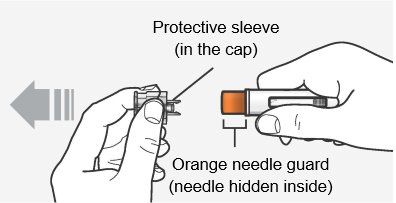
Step 6 – Pull off the cap
Hold the autoinjector body with 1 hand, and carefully pull the cap straight off with your other hand. Do not twist it off. The orange needle guard is now exposed and the needle is hidden underneath.
- Throw away (dispose of) the cap.
- Do not touch the needle or push on the orange needle guard with your finger.
- Do not recap the autoinjector. This could cause the medicine to come out too soon or damage the autoinjector.
Step 7 – Inject WAINUA using the autoinjector
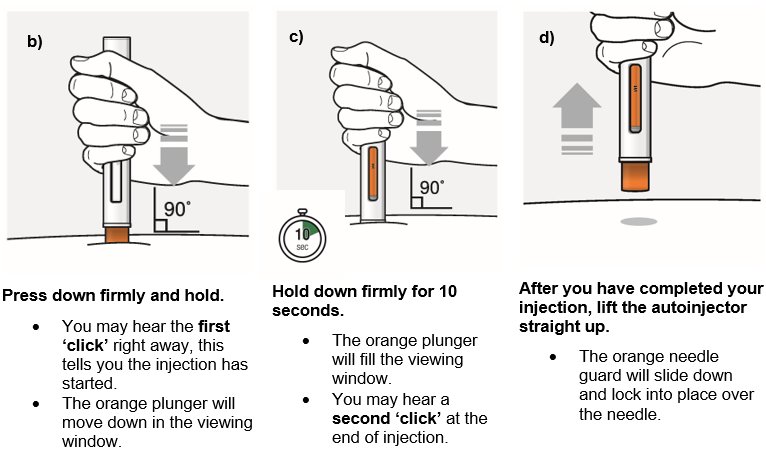
Inject the medicine using the autoinjector by following the steps in figures a, b, c and d.
When injecting, press and hold the autoinjector for 10 seconds until the orange plunger fills the viewing window. You may hear a first ‘click’ at the start of the injection and a second ‘click’ at the end of injection. This is normal.
Do not move or change the position of the autoinjector after the injection has started.
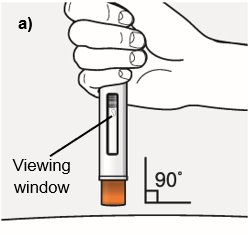
Position the autoinjector.
- Place the orange needle guard flat against your skin (90-degree angle).
- Make sure you can see the viewing window.
Step 8 – Check the viewing window
Check the viewing window to make sure all the medicine has been injected.
If the orange plunger rod does not fill the viewing window, you may not have received the full dose.
If this happens or if you have other concerns, contact your healthcare provider.

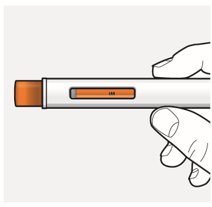
Step 9 – Check the injection site
There may be a small amount of blood or liquid where you injected. This is normal.
If needed, press a cotton ball or gauze on the area and apply a small bandage.
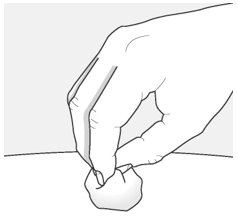
Step 10 – Throw away (dispose of) the used autoinjector
Put the used autoinjector in an FDA-cleared sharps disposal container right away after use.
Do not throw away the autoinjector in your household trash.
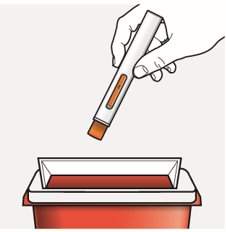
Disposal guidelines
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and autoinjectors. For more information about safe sharps disposal, and for specific information about sharps disposal in the area that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
Do not throw away (dispose of) your used sharps disposal container in your household trash unless your community guidelines permit this.
Do not recycle your used sharps disposal container.
For more information, go to https://www.wainua.com or call 1-800-236-9933. If you still have questions, contact your healthcare provider.
Distributed by: AstraZeneca Pharmaceuticals LP, Wilmington, DE 19850
©AstraZeneca 2025
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Revised: 12/2025
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC: 0310-9400-01 Rx only
WAINUA™ 45 mg/0.8 mL
(eplontersen) injection
for subcutaneous use
Each WAINUA autoinjector contains 45 mg eplontersen
(equivalent to 47 mg eplontersen sodium) in 0.8 mL of solution, for single-dose only.
Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton protected from light.
Do not freeze. Do not expose to heat.
1 single-dose autoinjector
IONIS™ AstraZeneca
-
INGREDIENTS AND APPEARANCE
WAINUA
eplontersen injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0310-9400 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPLONTERSEN (UNII: 0GRZ0F5XJ6) (EPLONTERSEN - UNII:0GRZ0F5XJ6) EPLONTERSEN 45 mg in 0.8 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE (UNII: 5QWK665956) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0310-9400-01 1 in 1 CARTON 12/21/2023 1 0.8 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA217388 12/21/2023 Labeler - AstraZeneca Pharmaceuticals LP (054743190)
Trademark Results [WAINUA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 WAINUA 98054331 not registered Live/Pending |
AstraZeneca AB 2023-06-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.