MICONAZOLE NITRATE 1%- micaonazole spray
Miconazole nitrate by
Drug Labeling and Warnings
Miconazole nitrate by is a Animal medication manufactured, distributed, or labeled by Butler Animal health Supply, LLC dba Covetrus North America, FIRST PRIORITY INCORPORATED. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- VETERINARY INDICATIONS
- PRECAUTIONS
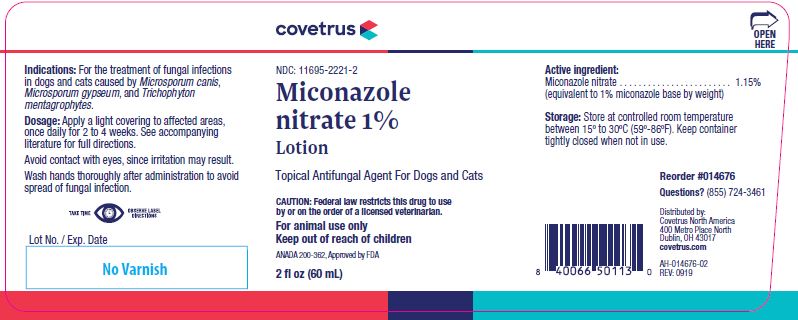
- Description:
- Indications:
- Precautions:
-
Dosage and administration:
Accurate diagnosis of the infecting organism is essential. Identification should be made either by direct microscopic examination of a mounting of infected tissue in a solution of potassium hydroxide, or by culture on an appropriate medium.
Lotion:
Apply a light covering of Miconazole nitrate 1% Lotion to affected areas, once daily, for 2 to 4 weeks. Application is best accomplished using a gauze pad or cotton swab.
Spray:
Spray affected areas from a distance of 2 to 4 inches to apply a light covering, once daily for 2 to 4 weeks. Do not allow pet to contact finished wood surfaces until pet is thoroughly dried.
Medication must be continued until the infecting organism is completely eradicated as indicated by appropriate clinical or laboratory examination. If no improvement is noticed within 2 weeks, diagnosis should be re-evaluated. Difficult cases may require treatment for 6 weeks.
General measures in regard to hygiene should be observed to control sources of infection or reinfection. Clipping of hair around and over the sites of infection should be done at the start of treatment and again as necessary. - How supplied:
-
Caution:
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Reorder #014676 (60 mL), 0104677 (120 mL) and 014678 (240 mL)
Questions? (855) 724-3461Distributed by:
Covetrus North America
400 Metro Place North
Dublin, OH 43017
covetrus.comANADA #200-362, Approved by FDA

60 mL:
AH-014676-02
REV: 0919120 mL:
AH-014677-02
REV: 1019240 mL:
AH-014678-02
REV: 1019 - PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MICONAZOLE NITRATE 1%
micaonazole sprayProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 11695-2221 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MICONAZOLE NITRATE (UNII: VW4H1CYW1K) (MICONAZOLE - UNII:7NNO0D7S5M) MICONAZOLE NITRATE 11.5 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 11695-2221-2 72 in 1 CASE 1 60 mL in 1 BOTTLE, DROPPER 2 NDC: 11695-2221-4 12 in 1 CASE 2 120 mL in 1 BOTTLE, SPRAY 3 NDC: 11695-2221-8 12 in 1 CASE 3 240 mL in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200362 10/01/2019 Labeler - Butler Animal health Supply, LLC dba Covetrus North America (603750329) Establishment Name Address ID/FEI Business Operations FIRST PRIORITY INCORPORATED 179925722 manufacture, label
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.