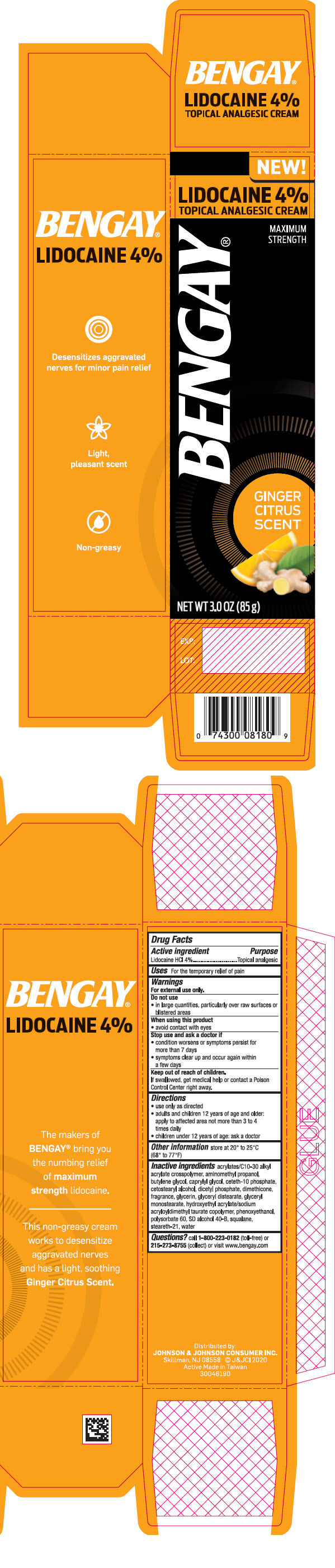
BENGAY GINGER CITRUS- lidocaine cream
BENGAY by
Drug Labeling and Warnings
BENGAY by is a Otc medication manufactured, distributed, or labeled by Johnson & Johnson Consumer Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
acrylates/C10-30 alkyl acrylate crosspolymer, aminomethyl propanol, butylene glycol, caprylyl glycol, ceteth-10 phosphate, cetostearyl alcohol, dicetyl phosphate, dimethicone, fragrance, glycerin, glyceryl distearate, glyceryl monostearate, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer, phenoxyethanol, polysorbate 60, SD alcohol 40-B, squalane, steareth-21, water
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 85 g Tube Carton
-
INGREDIENTS AND APPEARANCE
BENGAY GINGER CITRUS
lidocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69968-0615 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Lidocaine (UNII: 98PI200987) (Lidocaine - UNII:98PI200987) Lidocaine 40 mg in 1 g Inactive Ingredients Ingredient Name Strength CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) Butylene Glycol (UNII: 3XUS85K0RA) Caprylyl Glycol (UNII: 00YIU5438U) Ceteth-10 Phosphate (UNII: 4E05O5N49G) Cetostearyl Alcohol (UNII: 2DMT128M1S) DIHEXADECYL PHOSPHATE (UNII: 2V6E5WN99N) Dimethicone (UNII: 92RU3N3Y1O) Glycerin (UNII: PDC6A3C0OX) Glyceryl Distearate (UNII: 73071MW2KM) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) Phenoxyethanol (UNII: HIE492ZZ3T) Polysorbate 60 (UNII: CAL22UVI4M) ALCOHOL (UNII: 3K9958V90M) Squalane (UNII: GW89575KF9) Steareth-21 (UNII: 53J3F32P58) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69968-0615-3 1 in 1 CARTON 01/21/2020 1 85 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part348 01/21/2020 Labeler - Johnson & Johnson Consumer Inc (002347102)
Trademark Results [BENGAY]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BENGAY 88480389 not registered Live/Pending |
Johnson & Johnson 2019-06-19 |
 BENGAY 88480050 not registered Live/Pending |
Johnson & Johnson 2019-06-19 |
 BENGAY 88480042 not registered Live/Pending |
Johnson & Johnson 2019-06-19 |
 BENGAY 88480034 not registered Live/Pending |
Johnson & Johnson 2019-06-19 |
 BENGAY 88479433 not registered Live/Pending |
Johnson & Johnson 2019-06-19 |
 BENGAY 88479432 not registered Live/Pending |
Johnson & Johnson 2019-06-19 |
 BENGAY 88479428 not registered Live/Pending |
Johnson & Johnson 2019-06-19 |
 BENGAY 88479422 not registered Live/Pending |
Johnson & Johnson 2019-06-19 |
 BENGAY 88478298 not registered Live/Pending |
Johnson & Johnson 2019-06-18 |
 BENGAY 88477924 5926681 Live/Registered |
Johnson & Johnson 2019-06-18 |
 BENGAY 88477920 5926680 Live/Registered |
Johnson & Johnson 2019-06-18 |
 BENGAY 88477916 5926679 Live/Registered |
Johnson & Johnson 2019-06-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.