PIOGLITAZONE HYDROCHLORIDE tablet
Pioglitazone Hydrochloride by
Drug Labeling and Warnings
Pioglitazone Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by CELLTRION USA, INC., CELLTRION, INC., CELLTRION PHARM, INC., Cipla Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PIOGLITAZONE TABLETS safely and effectively. See full prescribing information for PIOGLITAZONE TABLETS.
PIOGLITAZONE tablets, for oral use
Initial U.S. Approval: 1999WARNING: CONGESTIVE HEART FAILURE
See full prescribing information for complete boxed warning.
- Thiazolidinediones, including pioglitazone tablets, cause or exacerbate congestive heart failure in some patients. (5.1)
- After initiation of pioglitazone tablets, and after dose increases, monitor patients carefully for signs and symptoms of heart failure (e.g., excessive, rapid weight gain, dyspnea, and/or edema). If heart failure develops, it should be managed according to current standards of care and discontinuation or dose reduction of pioglitazone tablets must be considered. (5.1)
- Pioglitazone tablets are not recommended in patients with symptomatic heart failure. (5.1)
- Initiation of pioglitazone tablets in patients with established New York Heart Association (NYHA) Class III or IV heart failure is contraindicated. (4, 5.1)
INDICATIONS AND USAGE
Pioglitazone tablets are a thiazolidinedione and an agonist for peroxisome proliferator-activated receptor (PPAR) gamma indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus in multiple clinical settings. (1, 14)
Important Limitations of Use:
- Not for treatment of type 1 diabetes or diabetic ketoacidosis. (1)
DOSAGE AND ADMINISTRATION
- Initiate pioglitazone tablets at 15 mg or 30 mg once daily. Limit initial dose to 15 mg once daily in patients with NYHA Class I or II heart failure. (2.1)
- If there is inadequate glycemic control, the dose can be increased in 15 mg increments up to a maximum of 45 mg once daily. (2.1)
- Obtain liver tests before starting pioglitazone tablets. If abnormal, use caution when treating with pioglitazone tablets, investigate the probable cause, treat (if possible) and follow appropriately. Monitoring liver tests while on pioglitazone tablets is not recommended in patients without liver disease. (5.3)
DOSAGE FORMS AND STRENGTHS
Tablets: 15 mg, 30 mg, and 45 mg (3)
CONTRAINDICATIONS
- Initiation in patients with established New York Heart Association (NYHA) Class III or IV heart failure [see Boxed Warning ]. (4)
- Use in patients with known hypersensitivity to pioglitazone or any other component of pioglitazone tablets. (4)
WARNINGS AND PRECAUTIONS
- Congestive heart failure: Fluid retention may occur and can exacerbate or lead to congestive heart failure. Combination use with insulin and use in congestive heart failure NYHA Class I and II may increase risk. Monitor patients for signs and symptoms. (5.1)
- Hypoglycemia: When used with insulin or an insulin secretagogue, a lower dose of the insulin or insulin secretagogue may be needed to reduce the risk of hypoglycemia. (5.2)
- Hepatic effects: Postmarketing reports of hepatic failure, sometimes fatal. Causality cannot be excluded. If liver injury is detected, promptly interrupt pioglitazone tablets and assess patient for probable cause, then treat cause if possible, to resolution or stabilization. Do not restart pioglitazone tablets if liver injury is confirmed and no alternate etiology can be found. (5.3)
- Bladder cancer: May increase the risk of bladder cancer. Do not use in patients with active bladder cancer. Use caution when using in patients with a prior history of bladder cancer. (5.4)
- Edema: Dose-related edema may occur. (5.5)
- Fractures: Increased incidence in female patients. Apply current standards of care for assessing and maintaining bone health. (5.6)
- Macular edema: Postmarketing reports. Recommend regular eye exams in all patients with diabetes according to current standards of care with prompt evaluation for acute visual changes. (5.7)
- Macrovascular outcomes: There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with pioglitazone tablets. (5.8)
ADVERSE REACTIONS
Most common adverse reactions (≥5%) are upper respiratory tract infection, headache, sinusitis, myalgia, and pharyngitis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact CELLTRION, INC. at 1-844-837-6511 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 4/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: CONGESTIVE HEART FAILURE
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommendations for All Patients
2.2 Concomitant Use with an Insulin Secretagogue or Insulin
2.3 Concomitant Use with Strong CYP2C8 Inhibitors
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Congestive Heart Failure
5.2 Hypoglycemia
5.3 Hepatic Effects
5.4 Urinary Bladder Tumors
5.5 Edema
5.6 Fractures
5.7 Macular Edema
5.8 Macrovascular Outcomes
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Strong CYP2C8 Inhibitors
7.2 CYP2C8 Inducers
7.3 Topiramate
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Monotherapy
14.2 Combination Therapy
16 HOW SUPPLIED/ STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: CONGESTIVE HEART FAILURE
WARNING: CONGESTIVE HEART FAILURE
- Thiazolidinediones, including pioglitazone tablets, cause or exacerbate congestive heart failure in some patients [see Warnings and Precautions (5.1)].
- After initiation of pioglitazone tablets, and after dose increases, monitor patients carefully for signs and symptoms of heart failure (e.g., excessive, rapid weight gain, dyspnea, and/or edema). If heart failure develops, it should be managed according to current standards of care and discontinuation or dose reduction of pioglitazone tablets must be considered.
- Pioglitazone tablets are not recommended in patients with symptomatic heart failure.
- Initiation of pioglitazone tablets in patients with established New York Heart Association (NYHA) Class III or IV heart failure is contraindicated [see 4 and Warnings and Precautions (5.1)].
-
1 INDICATIONS AND USAGE
Monotherapy and Combination Therapy
Pioglitazone tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus in multiple clinical settings [see Clinical Studies (14)].
Important Limitations of Use
Pioglitazone tablets exert their antihyperglycemic effect only in the presence of endogenous insulin. Pioglitazone tablets should not be used to treat type 1 diabetes or diabetic ketoacidosis, as it would not be effective in these settings.
Use caution in patients with liver disease [see Warnings and Precautions (5.3)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommendations for All Patients
Pioglitazone tablets should be taken once daily and can be taken without regard to meals.
The recommended starting dose for patients without congestive heart failure is 15 mg or 30 mg once daily.
The recommended starting dose for patients with congestive heart failure (NYHA Class I or II) is 15 mg once daily.
The dose can be titrated in increments of 15 mg up to a maximum of 45 mg once daily based on glycemic response as determined by HbA1c.
After initiation of pioglitazone tablets or with dose increase, monitor patients carefully for adverse reactions related to fluid retention such as weight gain, edema, and signs and symptoms of congestive heart failure [see Boxed Warning and Warnings and Precautions (5.5)].
Liver tests (serum alanine and aspartate aminotransferases, alkaline phosphatase, and total bilirubin) should be obtained prior to initiating pioglitazone tablets. Routine periodic monitoring of liver tests during treatment with pioglitazone tablets is not recommended in patients without liver disease. Patients who have liver test abnormalities prior to initiation of pioglitazone tablets or who are found to have abnormal liver tests while taking pioglitazone tablets should be managed as described under Warnings and Precautions [see Warnings and Precautions (5.3) and Clinical Pharmacology (12.3)].
2.2 Concomitant Use with an Insulin Secretagogue or Insulin
If hypoglycemia occurs in a patient co-administered pioglitazone tablets and an insulin secretagogue (e.g., sulfonylurea), the dose of the insulin secretagogue should be reduced.
If hypoglycemia occurs in a patient co-administered pioglitazone tablets and insulin, the dose of insulin should be decreased by 10% to 25%. Further adjustments to the insulin dose should be individualized based on glycemic response.
2.3 Concomitant Use with Strong CYP2C8 Inhibitors
Coadministration of pioglitazone tablets and gemfibrozil, a strong CYP2C8 inhibitor, increases pioglitazone exposure approximately 3-fold. Therefore, the maximum recommended dose of pioglitazone tablets is 15 mg daily when used in combination with gemfibrozil or other strong CYP2C8 inhibitors [see Drug Interactions (7.1) and Clinical Pharmacodynamic (12.3)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Initiation in patients with established NYHA Class III or IV heart failure [see Boxed Warning ].
Use in patients with known hypersensitivity to pioglitazone or any other component of pioglitazone tablets. -
5 WARNINGS AND PRECAUTIONS
5.1 Congestive Heart Failure
Pioglitazone tablets, like other thiazolidinediones, can cause dose-related fluid retention when used alone or in combination with other antidiabetic medications and is most common when pioglitazone tablets are used in combination with insulin. Fluid retention may lead to or exacerbate congestive heart failure. Patients should be observed for signs and symptoms of congestive heart failure. If congestive heart failure develops, it should be managed according to current standards of care and discontinuation or dose reduction of pioglitazone tablets must be considered [see Boxed Warning , Contraindications (4) , and Adverse Reactions (6.1) ].
5.2 Hypoglycemia
Patients receiving pioglitazone tablets in combination with insulin or other antidiabetic medications (particularly insulin secretagogues such as sulfonylureas) may be at risk for hypoglycemia. A reduction in the dose of the concomitant antidiabetic medication may be necessary to reduce the risk of hypoglycemia [see Dosage and Administration (2.2)].
5.3 Hepatic Effects
There have been postmarketing reports of fatal and non-fatal hepatic failure in patients taking pioglitazone tablets, although the reports contain insufficient information necessary to establish the probable cause. There has been no evidence of drug-induced hepatotoxicity in the pioglitazone tablets controlled clinical trial database to date [see Adverse Reactions (6.1)].
Patients with type 2 diabetes may have fatty liver disease or cardiac disease with episodic congestive heart failure, both of which may cause liver test abnormalities, and they may also have other forms of liver disease, many of which can be treated or managed. Therefore, obtaining a liver test panel (serum alanine aminotransferase [ALT], aspartate aminotransferase [AST], alkaline phosphatase, and total bilirubin) and assessing the patient is recommended before initiating pioglitazone tablets therapy. In patients with abnormal liver tests, pioglitazone tablets should be initiated with caution.
Measure liver tests promptly in patients who report symptoms that may indicate liver injury, including fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice. In this clinical context, if the patient is found to have abnormal liver tests (ALT greater than 3 times the upper limit of the reference range), pioglitazone tablets treatment should be interrupted and investigation done to establish the probable cause. Pioglitazone tablets should not be restarted in these patients without another explanation for the liver test abnormalities.
Patients who have serum ALT greater than three times the reference range with serum total bilirubin greater than two times the reference range without alternative etiologies are at risk for severe drug-induced liver injury, and should not be restarted on pioglitazone tablets. For patients with lesser elevations of serum ALT or bilirubin and with an alternate probable cause, treatment with pioglitazone tablets can be used with caution.
5.4 Urinary Bladder Tumors
Tumors were observed in the urinary bladder of male rats in the two-year carcinogenicity study [see Nonclinical Toxicology (13.1)]. In addition, during the three year PROactive clinical trial, 14 patients out of 2605 (0.54%) randomized to pioglitazone tablets and 5 out of 2633 (0.19%) randomized to placebo were diagnosed with bladder cancer. After excluding patients in whom exposure to study drug was less than one year at the time of diagnosis of bladder cancer, there were 6 (0.23%) cases on pioglitazone tablets and two (0.08%) cases on placebo. After completion of the trial, a large subset of patients was observed for up to 10 additional years, with little additional exposure to pioglitazone tablets. During the 13 years of both PROactive and observational follow-up, the occurrence of bladder cancer did not differ between patients randomized to pioglitazone tablets or placebo (HR =1.00; [95% CI: 0.59−1.72]).
Findings regarding the risk of bladder cancer in patients exposed to pioglitazone tablets vary among observational studies; some did not find an increased risk of bladder cancer associated with pioglitazone tablets, while others did.
A large prospective10-year observational cohort study conducted in the United States found no statistically significant increase in the risk of bladder cancer in diabetic patients ever exposed to pioglitazone tablets, compared to those never exposed to pioglitazone tablets (HR =1.06 [95% CI 0.89−1.26]).
A retrospective cohort study conducted with data from the United Kingdom found a statistically significant association between ever exposure to pioglitazone tablets and bladder cancer (HR: 1.63; [95% CI: 1.22−2.19]).
Associations between cumulative dose or cumulative duration of exposure to pioglitazone tablets and bladder cancer were not detected in some studies including the 10-year observational study in the U.S., but were in others. Inconsistent findings and limitations inherent in these and other studies preclude conclusive interpretations of the observational data.
Pioglitazone tablets may be associated with an increase in the risk of urinary bladder tumors. There are insufficient data to determine whether pioglitazone is a tumor promoter for urinary bladder tumors.
Consequently, pioglitazone tablets should not be used in patients with active bladder cancer and the benefits of glycemic control versus unknown risks for cancer recurrence with pioglitazone tablets should be considered in patients with a prior history of bladder cancer.
5.5 Edema
In controlled clinical trials, edema was reported more frequently in patients treated with pioglitazone tablets than in placebo-treated patients and is dose-related [see Adverse Reactions (6.1)]. In postmarketing experience, reports of new onset or worsening edema have been received.
Pioglitazone tablets should be used with caution in patients with edema. Because thiazolidinediones, including pioglitazone tablets, can cause fluid retention, which can exacerbate or lead to congestive heart failure, pioglitazone tablets should be used with caution in patients at risk for congestive heart failure. Patients treated with pioglitazone tablets should be monitored for signs and symptoms of congestive heart failure [see Boxed Warning , Warnings and Precautions (5.1) and Patient Counseling Information (17)].
5.6 Fractures
In PROactive (the Prospective Pioglitazone Clinical Trial in Macrovascular Events), 5238 patients with type 2 diabetes and a history of macrovascular disease were randomized to pioglitazone tablets (N=2605), force-titrated up to 45 mg daily or placebo (N=2633) in addition to standard of care. During a mean follow-up of 34.5 months, the incidence of bone fracture in females was 5.1% (44/870) for pioglitazone tablets versus 2.5% (23/905) for placebo. This difference was noted after the first year of treatment and persisted during the course of the study. The majority of fractures observed in female patients were nonvertebral fractures including lower limb and distal upper limb. No increase in the incidence of fracture was observed in men treated with pioglitazone tablets (1.7%) versus placebo (2.1%). The risk of fracture should be considered in the care of patients, especially female patients, treated with pioglitazone tablets and attention should be given to assessing and maintaining bone health according to current standards of care.
5.7 Macular Edema
Macular edema has been reported in postmarketing experience in diabetic patients who were taking pioglitazone tablets or another thiazolidinedione. Some patients presented with blurred vision or decreased visual acuity, but others were diagnosed on routine ophthalmologic examination.
Most patients had peripheral edema at the time macular edema was diagnosed. Some patients had improvement in their macular edema after discontinuation of the thiazolidinedione.
Patients with diabetes should have regular eye exams by an ophthalmologist according to current standards of care. Patients with diabetes who report any visual symptoms should be promptly referred to an ophthalmologist, regardless of the patient’s underlying medications or other physical findings [see Adverse Reactions (6.1)].
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed elsewhere in the labeling:
- Congestive heart failure [see Boxed Warning and Warnings and Precautions (5.1)]
- Edema [see Warnings and Precautions (5.5)]
- Fractures [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Over 8500 patients with type 2 diabetes have been treated with pioglitazone in randomized, double-blind, controlled clinical trials, including 2605 patients with type 2 diabetes and macrovascular disease treated with pioglitazone in the PROactive clinical trial. In these trials, over 6000 patients have been treated with pioglitazone for six months or longer, over 4500 patients have been treated with pioglitazone for one year or longer, and over 3000 patients have been treated with pioglitazone for at least two years.
In six pooled 16- to 26-week placebo-controlled monotherapy and 16- to 24-week add-on combination therapy trials, the incidence of withdrawals due to adverse events was 4.5% for patients treated with pioglitazone and 5.8% for comparator-treated patients. The most common adverse events leading to withdrawal were related to inadequate glycemic control, although the incidence of these events was lower (1.5%) with pioglitazone than with placebo (3.0%).
In the PROactive trial, the incidence of withdrawals due to adverse events was 9.0% for patients treated with pioglitazone and 7.7% for placebo-treated patients. Congestive heart failure was the most common serious adverse event leading to withdrawal occurring in 1.3% of patients treated with pioglitazone and 0.6% of patients treated with placebo.
Common Adverse Events: 16- to 26-Week Monotherapy Trials
A summary of the incidence and type of common adverse events reported in three pooled 16- to 26-week placebo-controlled monotherapy trials of pioglitazone is provided in Table 1. Terms that are reported represent those that occurred at an incidence of >5% and more commonly in patients treated with pioglitazone than in patients who received placebo. None of these adverse events were related to pioglitazone dose.
Table 1: Three Pooled 16- to 26-Week Placebo-Controlled Clinical Trials of Pioglitazone Monotherapy: Adverse Events Reported at an Incidence > 5% and More Commonly in Patients Treated with Pioglitazone than in Patients Treated with Placebo % of Patients Placebo
N=259Pioglitazone
N=606Upper Respiratory Tract Infection 8.5 13.2 Headache 6.9 9.1 Sinusitis 4.6 6.3 Myalgia 2.7 5.4 Pharyngitis 0.8 5.1 Common Adverse Events: 16- to 24-Week Add-on Combination Therapy Trials
A summary of the overall incidence and types of common adverse events reported in trials of pioglitazone add-on to sulfonylurea is provided in Table 2. Terms that are reported represent those that occurred at an incidence of >5% and more commonly with the highest tested dose of pioglitazone.
Table 2: 16- to 24-Week Clinical Trials of Pioglitazone Add-on to Sulfonylurea 16-Week Placebo-Controlled Trial
Adverse Events Reported in > 5% of Patients and More
Commonly in Patients Treated with Pioglitazone 30mg +
Sulfonylurea than in Patients Treated with
Placebo + Sulfonylurea% of Patients Placebo
+ Sulfonylurea
N=187Pioglitazone
15 mg
+ Sulfonylurea
N=184Pioglitazone
30 mg
+ Sulfonylurea
N=189Edema 2.1 1.6 12.7 Headache 3.7 4.3 5.3 Flatulence 0.5 2.7 6.3 Weight Increased 0 2.7 5.3 24-Week Non-Controlled Double-Blind Trial Adverse Events
Reported in > 5% of Patients and More Commonly in Patients
Treated with Pioglitazone 45 mg + Sulfonylurea than in
Patients Treated with Pioglitazone 30 mg + Sulfonylurea% of Patients Pioglitazone 30 mg
+ Sulfonylurea
N=351Pioglitazone 45 mg
+ Sulfonylurea
N=351Hypoglycemia 13.4 15.7 Edema 10.5 23.1 Upper Respiratory Tract Infection 12.3 14.8 Weight Increased 9.1 13.4 Urinary Tract Infection 5.7 6.8 Note: The preferred terms of edema peripheral, generalized edema, pitting edema and fluid retention were combined to form the aggregate term of “edema.”
A summary of the overall incidence and types of common adverse events reported in trials of pioglitazone add-on to metformin is provided in Table 3. Terms that are reported represent those that occurred at an incidence of >5% and more commonly with the highest tested dose of pioglitazone.
Table 3: 16- to 24-Week Clinical Trials of Pioglitazone Add-on to Metformin 16-Week Placebo-Controlled Trial
Adverse Events Reported in > 5% of
Patients and More Commonly in
Patients Treated with Pioglitazone +
Metformin than in Patients Treated
with Placebo + Metformin% of Patients Placebo
+ Metformin
N=160Pioglitazone 30 mg
+ Metformin
N=168Edema 2.5 6.0 Headache 1.9 6.0 24-Week Non-Controlled Double-
Blind Trial Adverse Events Reported
in > 5% of Patients and More
Commonly in Patients Treated with
Pioglitazone 45 mg + Metformin than in
Patients Treated with
Pioglitazone 30 mg + Metformin% of Patients Pioglitazone 30 mg
+ Metformin
N=411Pioglitazone 45 mg
+ Metformin
N=416Upper Respiratory Tract Infection 12.4 13.5 Edema 5.8 13.9 Headache 5.4 5.8 Weight Increased 2.9 6.7 Note: The preferred terms of edema peripheral, generalized edema, pitting edema and fluid retention were combined to form the aggregate term of “edema.”
Table 4 summarizes the incidence and types of common adverse events reported in trials of pioglitazone add-on to insulin. Terms that are reported represent those that occurred at an incidence of >5% and more commonly with the highest tested dose of pioglitazone.
Table 4: 16- to 24-Week Clinical Trials of Pioglitazone Add-on to Insulin 16-Week Placebo-Controlled Trial
Adverse Events Reported in > 5% of Patients and
More Commonly in Patients Treated with
Pioglitazone 30 mg + Insulin than in Patients Treated with Placebo + Insulin% of Patients Placebo
+Insulin
N=187Pioglitazone
15 mg
+ Insulin
N=191Pioglitazone
30 mg
+ Insulin
N=188Hypoglycemia 4.8 7.9 15.4 Edema 7.0 12.6 17.6 Upper Respiratory Tract Infection 9.6 8.4 14.9 Headache 3.2 3.1 6.9 Weight Increased 0.5 5.2 6.4 Back Pain 4.3 2.1 5.3 Dizziness 3.7 2.6 5.3 Flatulence 1.6 3.7 5.3 24-Week Non-Controlled Double-Blind Trial
Adverse Events Reported in > 5% of Patients and
More Commonly in Patients Treated with
Pioglitazone 45 mg + Insulin than in Patients Treatedwith Pioglitazone 30 mg + Insulin% of Patients Pioglitazone 30 mg
+ Insulin
N=345Pioglitazone 45 mg
+ Insulin
N=345Hypoglycemia 43.5 47.8 Edema 22.0 26.1 Weight Increased 7.2 13.9 Urinary Tract Infection 4.9 8.7 Diarrhea 5.5 5.8 Back Pain 3.8 6.4 Blood Creatine Phosphokinase Increased 4.6 5.5 Sinusitis 4.6 5.5 Hypertension 4.1 5.5 Note: The preferred terms of edema peripheral, generalized edema, pitting edema and fluid retention were combined to form the aggregate term of “edema.”
A summary of the overall incidence and types of common adverse events reported in the PROactive trial is provided in Table 5. Terms that are reported represent those that occurred at an incidence of >5% and more commonly in patients treated with pioglitazone than in patients who received placebo.
Table 5: PROactive Trial: Incidence and Types of Adverse Events Reported in > 5% of Patients Treated with Pioglitazone and More Commonly than Placebo % of Patients Placebo
N=2633Pioglitazone
N=2605Hypoglycemia 18.8 27.3 Edema 15.3 26.7 Cardiac Failure 6.1 8.1 Pain in Extremity 5.7 6.4 Back Pain 5.1 5.5 Chest Pain 5.0 5.1 Mean duration of patient follow-up was 34.5 months.
Congestive Heart Failure
A summary of the incidence of adverse events related to congestive heart failure is provided in Table 6 for the 16- to 24-week add-on to sulfonylurea trials, for the 16- to 24-week add-on to insulin trials, and for the 16- to 24-week add-on to metformin trials. None of the events were fatal.
Table 6: Treatment–Emergent Adverse Events of Congestive Heart Failure (CHF) Patients Treated with Pioglitazone or Placebo Added on to a Sulfonylurea Number (%) of Patients Placebo-Controlled Trial
(16 weeks)Non-Controlled Double-Blind Trial
(24 weeks)Placebo
+ Sulfonylurea
N=187Pioglitazone
15 mg
+ Sulfonylurea
N=184Pioglitazone
30 mg
+ Sulfonylurea
N=189Pioglitazone
30 mg
+ Sulfonylurea
N=351Pioglitazone
45 mg
+ Sulfonylurea
N=351At least one congestive heart failure event 2 (1.1%) 0 0 1 (0.3%) 6 (1.7%) Hospitalized 2 (1.1%) 0 0 0 2 (0.6%) Patients Treated with Pioglitazoneor Placebo Added on to Insulin Number (%) of Patients Placebo-Controlled Trial
(16 weeks)Non-Controlled
Double-Blind Trial
(24 weeks)Placebo
+ Insulin
N=187Pioglitazone
15 mg
+ Insulin
N=191Pioglitazone
30 mg
+ Insulin
N=188Pioglitazone
30 mg
+ Insulin
N=345Pioglitazone
45 mg
+ Insulin
N=345At least one congestive heart failure event 0 2 (1.0%) 2 (1.1%) 3 (0.9%) 5 (1.4%) Hospitalized 0 2 (1.0%) 1 (0.5%) 1 (0.3%) 3 (0.9%) Patients Treated with Pioglitazone or Placebo Added on to Metformin Number (%) of Patients Placebo-Controlled Trial
(16 weeks)Non-Controlled
Double-Blind Trial
(24 weeks)Placebo
+ Metformin
N=160Pioglitazone
30 mg
+ Metformin
N=168Pioglitazone
30 mg
+ Metformin
N=411Pioglitazone
45 mg
+ Metformin
N=416At least one congestive heart failure event 0 1 (0.6%) 0 1 (0.2%) Hospitalized 0 1 (0.6%) 0 1 (0.2%) Patients with type 2 diabetes and NYHA class II or early class III congestive heart failure were randomized to receive 24 weeks of double-blind treatment with either pioglitazone at daily doses of 30 mg to 45 mg (n=262) or glyburide at daily doses of 10 mg to 15 mg (n=256). A summary of the incidence of adverse events related to congestive heart failure reported in this study is provided in Table 7.
Table 7: Treatment–Emergent Adverse Events of Congestive Heart Failure (CHF) in Patients with NYHA Class II or III Congestive Heart Failure Treated with Pioglitazone or Glyburide Number (%) of Subjects Pioglitazone
N=262Glyburide
N=256Death due to cardiovascular causes (adjudicated) 5 (1.9%) 6 (2.3%) Overnight hospitalization for worsening CHF (adjudicated) 26 (9.9%) 12 (4.7%) Emergency room visit for CHF (adjudicated) 4 (1.5%) 3 (1.2%) Patients experiencing CHF progression during study 35 (13.4%) 21 (8.2%) Congestive heart failure events leading to hospitalization that occurred during the PROactive trial are summarized in Table 8.
Table 8: Treatment–Emergent Adverse Events of Congestive Heart Failure (CHF) in PROactive Trial Number (%) of Patients Placebo
N=2633Pioglitazone
N=2605At least one hospitalized congestive heart failure event 108 (4.1%) 149 (5.7%) Fatal 22 (0.8%) 25 (1.0%) Hospitalized, non-fatal 86 (3.3%) 124 (4.7%) Cardiovascular Safety
In the PROactive trial, 5238 patients with type 2 diabetes and a history of macrovascular disease were randomized to pioglitazone (N=2605), force-titrated up to 45 mg daily or placebo (N=2633) in addition to standard of care. Almost all patients (95%) were receiving cardiovascular medications (beta blockers, ACE inhibitors, angiotensin II receptor blockers, calcium channel blockers, nitrates, diuretics, aspirin, statins and fibrates). At baseline, patients had a mean age of 62 years, mean duration of diabetes of 9.5 years, and mean HbA1c of 8.1%. Mean duration of follow-up was 34.5 months.
The primary objective of this trial was to examine the effect of pioglitazone on mortality and macrovascular morbidity in patients with type 2 diabetes mellitus who were at high risk for macrovascular events. The primary efficacy variable was the time to the first occurrence of any event in a cardiovascular composite endpoint that included all-cause mortality, nonfatal myocardial infarction (MI) including silent MI, stroke, acute coronary syndrome, cardiac intervention including coronary artery bypass grafting or percutaneous intervention, major leg amputation above the ankle, and bypass surgery or revascularization in the leg. A total of 514 (19.7%) patients treated with pioglitazone and 572 (21.7%) placebo-treated patients experienced at least one event from the primary composite endpoint (hazard ratio 0.90; 95% Confidence Interval: 0.80, 1.02; p=0.10).
Although there was no statistically significant difference between pioglitazone and placebo for the three-year incidence of a first event within this composite, there was no increase in mortality or in total macrovascular events with pioglitazone. The number of first occurrences and total individual events contributing to the primary composite endpoint is shown in Table 9.
Table 9: PROactive: Number of First and Total Events for Each Component within the Cardiovascular Composite Endpoint Cardiovascular Events Placebo
N=2633Pioglitazone
N=2605First Events
n (%)Total events
nFirst Events
n (%)Total events
nAny event 572 (21.7) 900 514 (19.7) 803 All-cause mortality 122 (4.6) 186 110 (4.2) 177 Non-fatal myocardial infarction (MI) 118 (4.5) 157 105 (4.0) 131 Stroke 96 (3.6) 119 76 (2.9) 92 Acute coronary syndrome 63 (2.4) 78 42 (1.6) 65 Cardiac intervention (CABG/PCI) 101 (3.8) 240 101 (3.9) 195 Major leg amputation 15 (0.6) 28 9 (0.3) 28 Leg revascularization 57 (2.2) 92 71 (2.7) 115 CABG = coronary artery bypass grafting; PCI = percutaneous intervention
Weight Gain
Dose-related weight gain occurs when pioglitazone is used alone or in combination with other antidiabetic medications. The mechanism of weight gain is unclear but probably involves a combination of fluid retention and fat accumulation.
Tables 10 and 11 summarize the changes in body weight with pioglitazone and placebo in the 16- to 26-week randomized, double-blind monotherapy and 16- to 24-week combination add-on therapy trials and in the PROactive trial.
Table 10: Weight Changes (kg) from Baseline During Randomized, Double-Blind Clinical Trials Control Group
(Placebo)Pioglitazone
15 mgPioglitazone
30 mgPioglitazone
45 mgMedian
(25th/75th
percentile)Median
(25th/75th
percentile)Median
(25th/75th
percentile)Median
(25th/75th
percentile)Monotherapy
(16 to 26 weeks)-1.4 (-2.7/0.0)
N=2560.9 (-0.5/3.4)
N=791.0 (-0.9/3.4)
N=1882.6 (0.2/5.4)
N=79Combination Therapy
(16 to 24 weeks)Sulfonylurea -0.5 (-1.8/0.7)
N=1872.0 (0.2/3.2)
N=1833.1 (1.1/5.4)
N=5284.1 (1.8/7.3)
N=333Metformin -1.4 (-3.2/0.3)
N=160N/A 0.9 (-1.3/3.2)
N=5671.8 (-0.9/5.0)
N=407Insulin 0.2 (-1.4/1.4)
N=1822.3 (0.5/4.3)
N=1903.3 (0.9/6.3)
N=5224.1 (1.4/6.8)
N=338Table 11: Median Change in Body Weight in Patients Treated with Pioglitazone Versus Patients Treated with Placebo During the Double-Blind Treatment Period in the PROactive Trial Placebo Pioglitazone Median
(25th/75th
percentile)Median
(25th/75th
percentile)Change from baseline to final visit (kg) -0.5 (-3.3, 2.0)
N=2581+3.6 (0.0, 7.5)
N=2560Note: Median exposure for both pioglitazone and Placebo was 2.7 years.
Edema
Edema induced from taking pioglitazone is reversible when pioglitazone is discontinued. The edema usually does not require hospitalization unless there is coexisting congestive heart failure. A summary of the frequency and types of edema adverse events occurring in clinical investigations of pioglitazone is provided in Table 12.
Table 12: Adverse Events of Edema in Patients Treated with Pioglitazone Number (%) of Patients Placebo Pioglitazone
15 mgPioglitazone
30 mgPioglitazone
45 mgMonotherapy (16 to 26 weeks) 3 (1.2%)
N=2592 (2.5%)
N=8113 (4.7%)
N=27511 (6.5%)
N=169Combined Therapy
(16 to 24 weeks)Sulfonylurea 4 (2.1%)
N=1873 (1.6%)
N=18461 (11.3%)
N=54081 (23.1%)
N=351Metformin 4 (2.5%)
N=160N/A 34 (5.9%)
N=57958 (13.9%)
N=416Insulin 13 (7.0%)
N=18724 (12.6%)
N=191109 (20.5%)
N=53390 (26.1%)
N=345Note: The preferred terms of edema peripheral, generalized edema, pitting edema and fluid retention were combined to form the aggregate term of “edema.”
Table 13: Adverse Events of Edema in Patients in the PROactive Trial Number (%) of Patients Placebo
N=2633Pioglitazone
N=2605419 (15.9%) 712 (27.3%) Note: The preferred terms of edema peripheral, generalized edema, pitting edema and fluid retention were combined to form the aggregate term of “edema.”
Hepatic Effects
There has been no evidence of induced hepatotoxicity with pioglitazone in the pioglitazone controlled clinical trial database to date. One randomized, double-blind 3-year trial comparing pioglitazone to glyburide as add-on to metformin and insulin therapy was specifically designed to evaluate the incidence of serum ALT elevation to greater than three times the upper limit of the reference range, measured every eight weeks for the first 48 weeks of the trial then every 12 weeks thereafter. A total of 3/1051 (0.3%) patients treated with pioglitazone and 9/1046 (0.9%) patients treated with glyburide developed ALT values greater than three times the upper limit of the reference range. None of the patients treated with pioglitazone in the pioglitazone controlled clinical trial database to date have had a serum ALT greater than three times the upper limit of the reference range and a corresponding total bilirubin greater than two times the upper limit of the reference range, a combination predictive of the potential for severe drug-induced liver injury.
Hypoglycemia
In the pioglitazone clinical trials, adverse events of hypoglycemia were reported based on clinical judgment of the investigators and did not require confirmation with fingerstick glucose testing.
In the 16-week add-on to sulfonylurea trial, the incidence of reported hypoglycemia was 3.7% with pioglitazone 30 mg and 0.5% with placebo. In the 16-week add-on to insulin trial, the incidence of reported hypoglycemia was 7.9% with pioglitazone 15 mg, 15.4% with pioglitazone 30 mg, and 4.8% with placebo.
The incidence of reported hypoglycemia was higher with pioglitazone 45 mg compared to pioglitazone 30 mg in both the 24-week add-on to sulfonylurea trial (15.7% vs. 13.4%) and in the 24-week add-on to insulin trial (47.8% vs. 43.5%).
Three patients in these four trials were hospitalized due to hypoglycemia. All three patients were receiving pioglitazone 30 mg (0.9%) in the 24-week add-on to insulin trial. An additional 14 patients reported severe hypoglycemia (defined as causing considerable interference with patient’s usual activities) that did not require hospitalization. These patients were receiving pioglitazone 45 mg in combination with sulfonylurea (n=2) or pioglitazone 30 mg or 45 mg in combination with insulin (n=12).
Urinary Bladder Tumors
Tumors were observed in the urinary bladder of male rats in the two-year carcinogenicity study [see Nonclinical Toxicology (13.1)]. During the three year PROactive clinical trial, 14 patients out of 2605 (0.54%) randomized to pioglitazone and 5 out of 2633 (0.19%) randomized to placebo were diagnosed with bladder cancer. After excluding patients in whom exposure to study drug was less than one year at the time of diagnosis of bladder cancer, there were 6 (0.23%) cases on pioglitazone and two (0.08%) cases on placebo. After completion of the trial, a large subset of patients was observed for up to 10 additional years, with little additional exposure to pioglitazone. During the 13 years of both PROactive and observational follow-up, the occurrence of bladder cancer did not differ between patients randomized to pioglitazone or placebo (HR =1.00; 95% CI: 0.59-1.72) [see Warnings and Precautions (5.4)].
Laboratory Abnormalities
Hematologic Effects
Pioglitazone may cause decreases in hemoglobin and hematocrit. In placebo-controlled monotherapy trials, mean hemoglobin values declined by 2% to 4% in patients treated with pioglitazone compared with a mean change in hemoglobin of -1% to +1% in placebo-treated patients. These changes primarily occurred within the first 4 to 12 weeks of therapy and remained relatively constant thereafter. These changes may be related to increased plasma volume associated with pioglitazone therapy and are not likely to be associated with any clinically significant hematologic effects.
Creatine Phosphokinase
During protocol-specified measurement of serum creatine phosphokinase (CPK) in pioglitazone clinical trials, an isolated elevation in CPK to greater than 10 times the upper limit of the reference range was noted in nine (0.2%) patients treated with pioglitazone (values of 2150 to 11400 IU/L) and in no comparator-treated patients. Six of these nine patients continued to receive pioglitazone, two patients were noted to have the CPK elevation on the last day of dosing and one patient discontinued pioglitazone due to the elevation. These elevations resolved without any apparent clinical sequelae. The relationship of these events to pioglitazone therapy is unknown.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of pioglitazone. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- New onset or worsening diabetic macular edema with decreased visual acuity [see Warnings and Precautions (5.7)]
- Fatal and nonfatal hepatic failure [see Warnings and Precautions (5.3)].
Postmarketing reports of congestive heart failure have been reported in patients treated with pioglitazone, both with and without previously known heart disease and both with and without concomitant insulin administration.
In postmarketing experience, there have been reports of unusually rapid increases in weight and increases in excess of that generally observed in clinical trials. Patients who experience such increases should be assessed for fluid accumulation and volume-related events such as excessive edema and congestive heart failure [see Boxed Warning and Warnings and Precautions (5.1)].
-
7 DRUG INTERACTIONS
7.1 Strong CYP2C8 Inhibitors
An inhibitor of CYP2C8 (e.g., gemfibrozil) significantly increases the exposure (area under the serum concentration-time curve or AUC) and half-life (t1/2) of pioglitazone. Therefore, the maximum recommended dose of pioglitazone tablets is 15 mg daily if used in combination with gemfibrozil or other strong CYP2C8 inhibitors [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3) ].
7.2 CYP2C8 Inducers
An inducer of CYP2C8 (e.g., rifampin) may significantly decrease the exposure (AUC) of pioglitazone. Therefore, if an inducer of CYP2C8 is started or stopped during treatment with pioglitazone tablets, changes in diabetes treatment may be needed based on clinical response without exceeding the maximum recommended daily dose of 45 mg for pioglitazone tablets [see Clinical Pharmacology (12.3) ].
7.3 Topiramate
A decrease in the exposure of pioglitazone and its active metabolites were noted with concomitant administration of pioglitazone and topiramate [see Clinical Pharmacology (12.3)]. The clinical relevance of this decrease is unknown; however, when pioglitazone and topiramate are used concomitantly, monitor patients for adequate glycemic control.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited data with pioglitazone in pregnant women are not sufficient to determine a drug-associated risk for major birth defects or miscarriage. There are risks to the mother and fetus associated with poorly controlled diabetes in pregnancy [see Clinical Considerations].
In animal reproduction studies, no adverse developmental effects were observed when pioglitazone was administered to pregnant rats and rabbits during organogenesis at exposures up to 5- and 35-times the 45 mg clinical dose, respectively, based on body surface area [see Data].
The estimated background risk of major birth defects is 6-10% in women with pre-gestational diabetes with a HbA1c >7 and has been reported to be as high as 20-25% in women with a HbA1c >10. The estimated background risk of miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Poorly controlled diabetes in pregnancy increases the maternal risk for diabetic ketoacidosis, pre-eclampsia, spontaneous abortions, preterm delivery, still birth and delivery complications. Poorly controlled diabetes increases the fetal risk for major birth defects, still birth, and macrosomia related morbidity.
Data
Animal Data
Pioglitazone administered to pregnant rats during organogenesis did not cause adverse developmental effects at a dose of 20 mg/kg (~5-times the 45 mg clinical dose), but delayed parturition and reduced embryofetal viability at 40 and 80 mg/kg, or ≥9-times the 45 mg clinical dose, by body surface area. In pregnant rabbits administered pioglitazone during organogenesis, no adverse developmental effects were observed at 80 mg/kg (~35-times the 45 mg clinical dose), but reduced embryofetal viability at 160 mg/kg, or ~69-times the 45 mg clinical dose, by body surface area. When pregnant rats received pioglitazone during late gestation and lactation, delayed postnatal development, attributed to decreased body weight, occurred in offspring at maternal doses of 10 mg/kg and above or ≥2 times the 45 mg clinical dose, by body surface area.
8.2 Lactation
Risk Summary
There is no information regarding the presence of pioglitazone in human milk, the effects on the breastfed infant, or the effects on milk production. Pioglitazone is present in rat milk; however due to species-specific differences in lactation physiology, animal data may not reliably predict drug levels in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for pioglitazone and any potential adverse effects on the breastfed infant from pioglitazone or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Discuss the potential for unintended pregnancy with premenopausal women as therapy with pioglitazone, like other thiazolidinediones, may result in ovulation in some anovulatory women.
8.4 Pediatric Use
Safety and effectiveness of pioglitazone tablets in pediatric patients have not been established.
Pioglitazone is not recommended for use in pediatric patients based on adverse effects observed in adults, including fluid retention and congestive heart failure, fractures, and urinary bladder tumors [see Warnings and Precautions (5.1, 5.4, 5.5 and 5.6)].
8.5 Geriatric Use
A total of 92 patients (15.2%) treated with pioglitazone tablets in the three pooled 16- to 26-week double-blind, placebo-controlled, monotherapy trials were ≥65 years old and two patients (0.3%) were ≥75 years old. In the two pooled 16- to 24-week add-on to sulfonylurea trials, 201 patients (18.7%) treated with pioglitazone tablets were ≥65 years old and 19 (1.8%) were ≥75 years old. In the two pooled 16- to 24-week add-on to metformin trials, 155 patients (15.5%) treated with pioglitazone tablets were ≥65 years old and 19 (1.9%) were ≥75 years old. In the two pooled 16- to 24-week add-on to insulin trials, 272 patients (25.4%) treated with pioglitazone tablets were ≥65 years old and 22 (2.1%) were ≥75 years old.
In PROactive, 1068 patients (41.0%) treated with pioglitazone tablets were ≥65 years old and 42 (1.6%) were ≥75 years old.
In pharmacokinetic studies with pioglitazone, no significant differences were observed in pharmacokinetic parameters between elderly and younger patients [see Clinical Pharmacology (12.3) ].
Although clinical experiences have not identified differences in effectiveness and safety between the elderly (≥65 years) and younger patients, these conclusions are limited by small sample sizes for patients ≥75 years old.
-
10 OVERDOSAGE
During controlled clinical trials, one case of overdose with pioglitazone tablets was reported. A male patient took 120 mg per day for four days, then 180 mg per day for seven days. The patient denied any clinical symptoms during this period.
In the event of overdosage, appropriate supportive treatment should be initiated according to the patient’s clinical signs and symptoms.
-
11 DESCRIPTION
Pioglitazone tablets are a thiazolidinedione and an agonist for peroxisome proliferator-activated receptor (PPAR) gamma that contains an oral antidiabetic medication: pioglitazone.
Pioglitazone [(±)-5-[[4-[2-(5-ethyl-2-pyridinyl) ethoxy] phenyl] methyl]-2,4-] thiazolidinedione monohydrochloride contains one asymmetric carbon, and the compound is synthesized and used as the racemic mixture. The two enantiomers of pioglitazone interconvert in vivo. No differences were found in the pharmacologic activity between the two enantiomers. The structural formula is as shown:
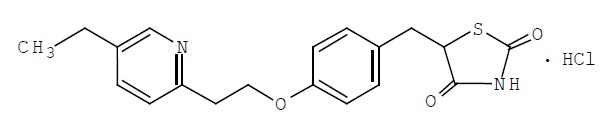
Pioglitazone hydrochloride is an odorless white crystalline powder that has a molecular formula of C19H20N2O3SHCl and a molecular weight of 392.90 daltons. It is soluble in N,N-dimethylformamide, slightly soluble in anhydrous ethanol, very slightly soluble in acetone and acetonitrile, practically insoluble in water, and insoluble in ether.
Pioglitazone tablets USP are available as a tablet for oral administration containing 15 mg, 30 mg, or 45 mg of pioglitazone (as the base) formulated with the following excipients: lactose monohydrate, carboxymethylcellulose calcium, hydroxypropylcellulose, and magnesium stearate.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Pioglitazone tablets are a thiazolidinedione that depends on the presence of insulin for its mechanism of action. Pioglitazone tablets decreases insulin resistance in the periphery and in the liver resulting in increased insulin-dependent glucose disposal and decreased hepatic glucose output. Pioglitazone is not an insulin secretagogue. Pioglitazone is an agonist for peroxisome proliferator-activated receptor-gamma (PPARγ). PPAR receptors are found in tissues important for insulin action such as adipose tissue, skeletal muscle, and liver. Activation of PPARγ nuclear receptors modulates the transcription of a number of insulin responsive genes involved in the control of glucose and lipid metabolism.
In animal models of diabetes, pioglitazone reduces the hyperglycemia, hyperinsulinemia, and hypertriglyceridemia characteristic of insulin-resistant states such as type 2 diabetes. The metabolic changes produced by pioglitazone result in increased responsiveness of insulin-dependent tissues and are observed in numerous animal models of insulin resistance.
Because pioglitazone enhances the effects of circulating insulin (by decreasing insulin resistance), it does not lower blood glucose in animal models that lack endogenous insulin.
12.2 Pharmacodynamics
Clinical studies demonstrate that pioglitazone tablets improve insulin sensitivity in insulin-resistant patients. Pioglitazone tablets enhance cellular responsiveness to insulin, increases insulin-dependent glucose disposal and improves hepatic sensitivity to insulin. In patients with type 2 diabetes, the decreased insulin resistance produced by pioglitazone tablets results in lower plasma glucose concentrations, lower plasma insulin concentrations, and lower HbA1c values. In controlled clinical trials, pioglitazone tablets had an additive effect on glycemic control when used in combination with a sulfonylurea, metformin, or insulin [see Clinical Studies (14.2)].
Patients with lipid abnormalities were included in clinical trials with pioglitazone tablets. Overall, patients treated with pioglitazone tablets had mean decreases in serum triglycerides, mean increases in HDL cholesterol, and no consistent mean changes in LDL and total cholesterol. There is no conclusive evidence of macrovascular benefit with pioglitazone tablets [see Warnings and Precautions (5.8) and Adverse Reactions (6.1)].
In a 26-week, placebo-controlled, dose-ranging monotherapy study, mean serum triglycerides decreased in the 15 mg, 30 mg, and 45 mg pioglitazone tablets dose groups compared to a mean increase in the placebo group. Mean HDL cholesterol increased to a greater extent in patients treated with pioglitazone tablets than in the placebo-treated patients. There were no consistent differences for LDL and total cholesterol in patients treated with pioglitazone tablets compared to placebo (see Table 14).
Table 14. Lipids in a 26-Week Placebo-Controlled Monotherapy Dose-Ranging Study Placebo Pioglitazone Tablets
15 mg
Once DailyPioglitazone Tablets
30 mg
Once DailyPioglitazone Tablets
45 mg
Once DailyTriglycerides (mg/dL) N=79 N=79 N=84 N=77 Baseline (mean) 263 284 261 260 Percent change from baseline (adjusted mean*) 4.8% -9.0%† -9.6%† -9.3%† HDL Cholesterol (mg/dL) N=79 N=79 N=83 N=77 Baseline (mean) 42 40 41 41 Percent change from baseline (adjusted mean*) 8.1% 14.1%† 12.2% 19.1%† LDL Cholesterol (mg/dL) N=65 N=63 N=74 N=62 Baseline (mean) 139 132 136 127 Percent change from baseline (adjusted mean*) 4.8% 7.2% 5.2% 6.0% Total Cholesterol (mg/dL) N=79 N=79 N=84 N=77 Baseline (mean) 225 220 223 214 Percent change from baseline (adjusted mean*) 4.4% 4.6% 3.3% 6.4% *Adjusted for baseline, pooled center, and pooled center by treatment interaction
†p < 0.05 versus placebo
In the two other monotherapy studies (16 weeks and 24 weeks) and in combination therapy studies with sulfonylurea (16 weeks and 24 weeks), metformin (16 weeks and 24 weeks) or insulin (16 weeks and 24 weeks), the results were generally consistent with the data above.
12.3 Pharmacokinetics
Following once-daily administration of pioglitazone tablets, steady-state serum concentrations of both pioglitazone and its major active metabolites, M-III (keto derivative of pioglitazone) and M-IV (hydroxyl derivative of pioglitazone), are achieved within seven days. At steady-state, M-III and M-IV reach serum concentrations equal to or greater than that of pioglitazone. At steady-state, in both healthy volunteers and patients with type 2 diabetes, pioglitazone comprises approximately 30% to 50% of the peak total pioglitazone serum concentrations (pioglitazone plus active metabolites) and 20% to 25% of the total AUC.
Cmax, AUC, and trough serum concentrations (Cmin) for pioglitazone and M-III and M-IV, increased proportionally with administered doses of 15 mg and 30 mg per day.
Absorption
Following oral administration of pioglitazone, Tmax of pioglitazone was within two hours. Food delays the Tmax to three to four hours but does not alter the extent of absorption (AUC).
Distribution
The mean apparent volume of distribution (Vd/F) of pioglitazone following single-dose administration is 0.63 ± 0.41 (mean ± SD) L/kg of body weight. Pioglitazone is extensively protein bound (>99%) in human serum, principally to serum albumin. Pioglitazone also binds to other serum proteins, but with lower affinity. M-III and M-IV are also extensively bound (>98%) to serum albumin.
Metabolism
Pioglitazone is extensively metabolized by hydroxylation and oxidation; the metabolites also partly convert to glucuronide or sulfate conjugates. Metabolites M-III and M-IV are the major circulating active metabolites in humans.
In vitro data demonstrate that multiple CYP isoforms are involved in the metabolism of pioglitazone, which include CYP2C8 and, to a lesser degree, CYP3A4 with additional contributions from a variety of other isoforms including the mainly extrahepatic CYP1A1. In vivo study of pioglitazone in combination with gemfibrozil, a strong CYP2C8 inhibitor, showed that pioglitazone is a CYP2C8 substrate [see Dosage and Administration (2.3) and Drug Interactions (7)]. Urinary 6β-hydroxycortisol/cortisol ratios measured in patients treated with pioglitazone tablets showed that pioglitazone is not a strong CYP3A4 enzyme inducer.
Excretion and Elimination
Following oral administration, approximately 15% to 30% of the pioglitazone dose is recovered in the urine. Renal elimination of pioglitazone is negligible, and the drug is excreted primarily as metabolites and their conjugates. It is presumed that most of the oral dose is excreted into the bile either unchanged or as metabolites and eliminated in the feces.
The mean serum half-life (t1/2) of pioglitazone and its metabolites (M-III and M-IV) range from three to seven hours and 16 to 24 hours, respectively. Pioglitazone has an apparent clearance, CL/F, calculated to be five to seven L/hr.
Renal Impairment
The serum elimination half-life of pioglitazone, M-III, and M-IV remains unchanged in patients with moderate (creatinine clearance [CLcr] 30 to 50 mL/min) and severe (CLcr <30 mL/min) renal impairment when compared to subjects with normal renal function. Therefore, no dose adjustment in patients with renal impairment is required.
Hepatic Impairment
Compared with healthy controls, subjects with impaired hepatic function (Child-Turcotte-Pugh Grade B/C) have an approximate 45% reduction in pioglitazone and total pioglitazone (pioglitazone, M-III, and M-IV) mean Cmax but no change in the mean AUC values. Therefore, no dose adjustment in patients with hepatic impairment is required.
There are postmarketing reports of liver failure with pioglitazone tablets and clinical trials have generally excluded patients with serum ALT >2.5 times the upper limit of the reference range. Use caution in patients with liver disease [see Warnings and Precautions (5.3)].
Geriatric Patients
In healthy elderly subjects, Cmax of pioglitazone was not significantly different, but AUC values were approximately 21% higher than those achieved in younger subjects. The mean t1/2 of pioglitazone was also prolonged in elderly subjects (about ten hours) as compared to younger subjects (about seven hours). These changes were not of a magnitude that would be considered clinically relevant.
Pediatric Patients
Safety and efficacy of pioglitazone in pediatric patients have not been established. Pioglitazone tablets are not recommended for use in pediatric patients [see Use in Specific Populations (8.4)].
Gender
The mean Cmax and AUC values of pioglitazone were increased 20% to 60% in women compared to men. In controlled clinical trials, HbA1c decreases from baseline were generally greater for females than for males (average mean difference in HbA1c 0.5%). Because therapy should be individualized for each patient to achieve glycemic control, no dose adjustment is recommended based on gender alone.
Ethnicity
Pharmacokinetic data among various ethnic groups are not available.
Drug-Drug Interactions
Table 15: Effect of Pioglitazone Coadministration on Systemic Exposure of Other Drugs Coadministered Drug Pioglitazone Dosage
Regimen (mg)*Name and Dose Regimens Change
in AUC†Change
in Cmax†45 mg
(N = 12)Warfarin‡ Daily loading then maintenance doses based PT and INR values
Quick’s Value = 35 ± 5%R-Warfarin ↓ 3% R-Warfarin ↓ 2% S-Warfarin ↓ 1% S-Warfarin ↑1% 45 mg
(N = 12)Digoxin 0.200 mg twice daily (loading dose) then 0.250 mg daily (maintenance dose, 7 days) ↑ 15% ↑ 17% 45 mg daily
for 21 days
(N = 35)Oral Contraceptive [Ethinyl Estradiol (EE) 0.035 mg plus
Norethindrone (NE) 1 mg] for 21 daysEE ↓ 11% EE ↓ 13% NE ↑ 3% NE ↓ 7% 45 mg
(N = 23)Fexofenadine 60 mg twice daily for 7 days ↑ 30% ↑ 37% 45 mg
(N = 14)Glipizide 5 mg daily for 7 days ↓ 3% ↓ 8% 45 mg daily
for 8 days
(N = 16)Metformin 1000 mg single dose on Day 8 ↓ 3% ↓ 5% 45 mg
(N = 21)Midazolam 7.5 mg single dose on Day 15 ↓ 26% ↓ 26% 45 mg
(N = 24)Ranitidine 150 mg twice daily for 7 days ↑ 1% ↓1% 45 mg daily
for 4 days
(N = 24)Nifedipine ER 30 mg daily for 4 days ↓ 13% ↓ 17% 45 mg
(N = 25)Atorvastatin Ca 80 mg daily for 7 days ↓ 14% ↓ 23% 45 mg
(N = 22)Theophylline 400 mg twice daily for 7 days ↑ 2% ↑ 5% *Daily for 7 days unless otherwise noted
†% change (with/without coadministered drug and no change = 0%); symbols of ↑ and ↓ indicate the exposure increase and decrease, respectively.
‡Pioglitazone had no clinically significant effect on prothrombin time
Table 16: Effect of Coadministered Drugs on Pioglitazone Systemic Exposure Coadministered Drug and Dosage
RegimenPioglitazone Dose
Regimen
(mg)*Change
in AUC†Change
in Cmax†Gemfibrozil 600 mg
twice daily for 2 days
(N = 12)15 mg
single dose↑ 3.2-fold‡ ↑ 6% Ketoconazole 200 mg
twice daily for 7 days
(N = 28)45 mg ↑ 34% ↑ 14% Rifampin 600 mg
daily for 5 days
(N = 10)30 mg
single dose↓ 54% ↓ 5% Fexofenadine 60 mg
twice daily for 7 days
(N = 23)45 mg ↑ 1% 0% Ranitidine 150 mg
twice daily for 4 days
(N = 23)45 mg ↓ 13% ↓ 16% Nifedipine ER 30 mg
daily for 7 days
(N = 23)45 mg ↑ 5% ↑ 4% Atorvastatin Ca 80 mg
daily for 7 days
(N = 24)45 mg ↓ 24% ↓ 31% Theophylline 400 mg
twice daily for 7 days
(N = 22)45 mg ↓ 4% ↓ 2% Topiramate 96 mg
twice daily for 7 days §
(N=26)30 mg § ↓ 15% ¶ 0% *Daily for 7 days unless otherwise noted
†Mean ratio (with/without coadministered drug and no change = 1-fold) % change (with/without coadministered drug and no change = 0%); symbols of ↑ and ↓ indicate the exposure increase and decrease, respectively.
‡The half-life of pioglitazone increased from 8.3 hours to 22.7 hours in the presence of gemfibrozil [see Dosage and Administration (2.3) and Drug Interactions (7.1)]
§Indicates duration of concomitant administration with highest twice-daily dose of topiramate from Day 14 onwards over the 22 days of study
¶Additional decrease in active metabolites; 60% for M-III and 16% for M-IV
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A two-year carcinogenicity study was conducted in male and female rats at oral doses up to 63 mg/kg (approximately 14 times the maximum recommended human oral dose of 45 mg based on mg/m2). Drug-induced tumors were not observed in any organ except for the urinary bladder of male rats. Benign and/or malignant transitional cell neoplasms were observed in male rats at 4 mg/kg/day and above (approximately equal to the maximum recommended human oral dose based on mg/m2). Urinary calculi with subsequent irritation and hyperplasia were postulated as the mechanism for bladder tumors observed in male rats. A two-year mechanistic study in male rats utilizing dietary acidification to reduce calculi formation was completed in 2009. Dietary acidification decreased but did not abolish the hyperplastic changes in the bladder. The presence of calculi exacerbated the hyperplastic response to pioglitazone but was not considered the primary cause of the hyperplastic changes.
The relevance to humans of the bladder findings in the male rat cannot be excluded.
A two-year carcinogenicity study was also conducted in male and female mice at oral doses up to 100 mg/kg/day (approximately 11 times the maximum recommended human oral dose based on mg/m2). No drug-induced tumors were observed in any organ.
Pioglitazone hydrochloride was not mutagenic in a battery of genetic toxicology studies, including the Ames bacterial assay, a mammalian cell forward gene mutation assay (CHO/HPRT and AS52/XPRT), an in vitro cytogenetics assay using CHL cells, an unscheduled DNA synthesis assay, and an in vivo micronucleus assay.
No adverse effects upon fertility were observed in male and female rats at oral doses up to 40 mg/kg pioglitazone hydrochloride daily prior to and throughout mating and gestation (approximately nine times the maximum recommended human oral dose based on mg/m2).
13.2 Animal Toxicology and/or Pharmacology
Heart enlargement has been observed in mice (100 mg/kg), rats (4 mg/kg and above) and dogs (3 mg/kg) treated orally with pioglitazone hydrochloride (approximately 11, 1, and 2 times the maximum recommended human oral dose for mice, rats, and dogs, respectively, based on mg/m2). In a one-year rat study, drug-related early death due to apparent heart dysfunction occurred at an oral dose of 160 mg/kg/day (approximately 35 times the maximum recommended human oral dose based on mg/m2). Heart enlargement was seen in a 13-week study in monkeys at oral doses of 8.9 mg/kg and above (approximately four times the maximum recommended human oral dose based on mg/m2), but not in a 52-week study at oral doses up to 32 mg/kg (approximately 13 times the maximum recommended human oral dose based on mg/m2).
-
14 CLINICAL STUDIES
14.1 Monotherapy
Three randomized, double-blind, placebo-controlled trials with durations from 16 to 26 weeks were conducted to evaluate the use of pioglitazone tablets as monotherapy in patients with type 2 diabetes. These trials examined pioglitazone tablets at doses up to 45 mg or placebo once daily in a total of 865 patients.
In a 26-week dose-ranging monotherapy trial, 408 patients with type 2 diabetes were randomized to receive 7.5 mg, 15 mg, 30 mg, or 45 mg of pioglitazone tablets, or placebo once daily. Therapy with any previous antidiabetic agent was discontinued eight weeks prior to the double-blind period. Treatment with 15 mg, 30 mg, and 45 mg of pioglitazone tablets produced statistically significant improvements in HbA1c and fasting plasma glucose (FPG) at endpoint compared to placebo (see Figure 1, Table 17).
Figure 1 shows the time course for changes in HbA1c in this 26-week study.
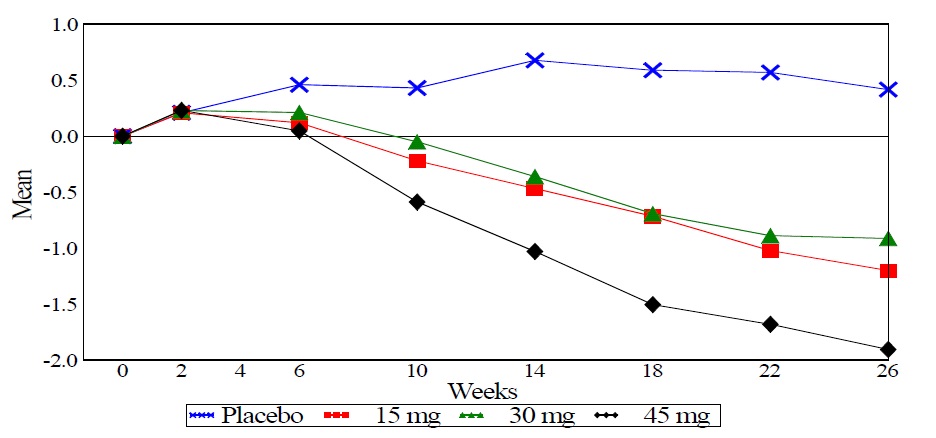
Figure 1 Mean Change from Baseline for HbA1c in a 26-Week Placebo-Controlled Dose-Ranging Study (Observed Values)
Table 17: Glycemic Parameters in a 26-Week Placebo-Controlled Dose-Ranging Monotherapy Trial Placebo Pioglitazone Tablets
15 mg
Once DailyPioglitazone Tablets
30 mg
Once DailyPioglitazone Tablets
45 mg
Once DailyTotal Population HbA1c (%) N=79 N=79 N=85 N=76 Baseline (mean) 10.4 10.2 10.2 10.3 Change from baseline (adjusted mean*) 0.7 -0.3 -0.3 -0.9 Difference from placebo (adjusted mean*)
95% Confidence Interval-1.0†
(-1.6, -0.4)-1.0†
(-1.6, -0.4)-1.6†
(-2.2, -1.0)Fasting Plasma Glucose (mg/dL) N=79 N=79 N=84 N=77 Baseline (mean) 268 267 269 276 Change from baseline (adjusted mean*) 9 -30 -32 -56 Difference from placebo (adjusted mean*)
95% Confidence Interval-39†
(-63, -16)-41†
(-64, -18)-65†
(-89, -42)*Adjusted for baseline, pooled center, and pooled center by treatment interaction
†p ≤ 0.05 vs. placebo
In a 24-week placebo-controlled monotherapy trial, 260 patients with type 2 diabetes were randomized to one of two forced-titration pioglitazone tablets treatment groups or a mock-titration placebo group. Therapy with any previous antidiabetic agent was discontinued six weeks prior to the double-blind period. In one pioglitazone tablets treatment group, patients received an initial dose of 7.5 mg once daily. After four weeks, the dose was increased to 15 mg once daily and after another four weeks, the dose was increased to 30 mg once daily for the remainder of the trial (16 weeks). In the second pioglitazone tablets treatment group, patients received an initial dose of 15 mg once daily and were titrated to 30 mg once daily and 45 mg once daily in a similar manner. Treatment with pioglitazone tablets, as described, produced statistically significant improvements in HbA1c and FPG at endpoint compared to placebo (see Table 18).
Table 18: Glycemic Parameters in a 24-Week Placebo-Controlled Forced-Titration Monotherapy Trial Placebo Pioglitazone Tablets
30 mg*
Once DailyPioglitazone Tablets
45 mg*
Once DailyTotal Population HbA1c (%) N=83 N=85 N=85 Baseline (mean) 10.8 10.3 10.8 Change from baseline (adjusted mean†) 0.9 -0.6 -0.6 Difference from placebo (adjusted mean†)
95% Confidence Interval-1.5‡
(-2.0, -1.0)-1.5‡
(-2.0, -1.0)Fasting Plasma Glucose (mg/dL) N=78 N=82 N=85 Baseline (mean) 279 268 281 Change from baseline (adjusted mean†) 18 -44 -50 Difference from placebo (adjusted mean†)
95% Confidence Interval-62‡
(-82, -0.41)-68‡
(-88, -0.48)*Final dose in forced titration
†Adjusted for baseline, pooled center, and pooled center by treatment interaction
‡p ≤ 0.05 vs. placebo
In a 16-week monotherapy trial, 197 patients with type 2 diabetes were randomized to treatment with 30 mg of pioglitazone tablets or placebo once daily. Therapy with any previous antidiabetic agent was discontinued six weeks prior to the double-blind period. Treatment with 30 mg of pioglitazone tablets produced statistically significant improvements in HbA1c and FPG at endpoint compared to placebo (see Table 19).
Table 19: Glycemic Parameters in a 16-Week Placebo-Controlled Monotherapy Trial Placebo Pioglitazone
Tablets
30 mg
Once DailyTotal Population HbA1c (%) N=93 N=100 Baseline (mean) 10.3 10.5 Change from baseline (adjusted mean*) 0.8 -0.6 Difference from placebo (adjusted mean*)
95% Confidence Interval-1.4†
(-1.8, -0.9)Fasting Plasma Glucose (mg/dL) N=91 N=99 Baseline (mean) 270 273 Change from baseline (adjusted mean*) 8 -50 Difference from placebo (adjusted mean*)
95% Confidence Interval-58†
(-77, -38)*Adjusted for baseline, pooled center, and pooled center by treatment interaction
†p ≤ 0.050 vs. placebo
14.2 Combination Therapy
Three 16-week, randomized, double-blind, placebo-controlled clinical trials were conducted to evaluate the effects of pioglitazone tablets (15 mg and/or 30 mg) on glycemic control in patients with type 2 diabetes who were inadequately controlled (HbA1c ≥8%) despite current therapy with a sulfonylurea, metformin, or insulin. In addition, three 24-week randomized, double-blind clinical trials were conducted to evaluate the effects of pioglitazone tablets 30 mg vs. pioglitazone tablets 45 mg on glycemic control in patients with type 2 diabetes who were inadequately controlled (HbA1c ≥8%) despite current therapy with a sulfonylurea, metformin, or insulin. Previous diabetes treatment may have been monotherapy or combination therapy.
Add-on to Sulfonylurea Trials
Two clinical trials were conducted with pioglitazone tablets in combination with a sulfonylurea. Both studies included patients with type 2 diabetes on any dose of a sulfonylurea, either alone or in combination with another antidiabetic agent. All other antidiabetic agents were withdrawn at least three weeks prior to starting study treatment.
In the first study, 560 patients were randomized to receive 15 mg or 30 mg of pioglitazone tablets or placebo once daily for 16 weeks in addition to their current sulfonylurea regimen. Treatment with pioglitazone tablets as add-on to sulfonylurea produced statistically significant improvements in HbA1c and FPG at endpoint compared to placebo add-on to sulfonylurea (see Table 20).
Table 20: Glycemic Parameters in a 16-Week Placebo-Controlled, Add-on to Sulfonylurea Trial Placebo
+ SulfonylureaPioglitazone Tablets 15 mg
+ SulfonylureaPioglitazone Tablets 30 mg
+ SulfonylureaTotal Population HbA1c (%) N=181 N=176 N=182 Baseline (mean) 9.9 10.0 9.9 Change from baseline (adjusted mean*) 0.1 -0.8 -1.2 Difference from placebo + sulfonylurea (adjusted mean*)
95% Confidence Interval-0.9†
(-1.2, -0.6)-1.3†
(-1.6, -1.0)Fasting Plasma Glucose (mg/dL) N=182 N=179 N=186 Baseline (mean) 236 247 239 Change from baseline (adjusted mean*) 6 -34 -52 Difference from placebo + sulfonylurea (adjusted mean*)
95% Confidence Interval-39†
(-52, -27)-58†
(-70, -46)*Adjusted for baseline, pooled center, and pooled center by treatment interaction
†p ≤ 0.05 vs. placebo + sulfonylurea
In the second trial, 702 patients were randomized to receive 30 mg or 45 mg of pioglitazone tablets once daily for 24 weeks in addition to their current sulfonylurea regimen. The mean reduction from baseline at Week 24 in HbA1c was 1.6% for the 30 mg dose and 1.7% for the 45 mg dose (see Table 21). The mean reduction from baseline at Week 24 in FPG was 52 mg/dL for the 30 mg dose and 56 mg/dL for the 45 mg dose.
The therapeutic effect of pioglitazone tablets in combination with sulfonylurea was observed in patients regardless of the sulfonylurea dose.
Table 21: Glycemic Parameters in a 24-Week Add-on to Sulfonylurea Trial Pioglitazone Tablets
30 mg +
SulfonylureaPioglitazone Tablets
45 mg +
SulfonylureaTotal Population HbA1c (%) N=340 N=332 Baseline (mean) 9.8 9.9 Change from baseline (adjusted mean*) -1.6 -1.7 Difference from 30 mg daily pioglitazone tablets + sulfonylurea (adjusted mean*) (95% CI) -0.1
(-0.4, 0.1)Fasting Plasma Glucose (mg/dL) N=338 N=329 Baseline (mean) 214 217 Change from baseline (adjusted mean*) -52 -56 Difference from 30 mg daily pioglitazone tablets + sulfonylurea (adjusted mean*) (95% CI) -5
(-12, 3)95% CI = 95% confidence interval
*Adjusted for baseline, pooled center, and pooled center by treatment interaction
Add-on to Metformin Trials
Two clinical trials were conducted with pioglitazone tablets in combination with metformin. Both trials included patients with type 2 diabetes on any dose of metformin, either alone or in combination with another antidiabetic agent. All other antidiabetic agents were withdrawn at least three weeks prior to starting study treatment.
In the first trial, 328 patients were randomized to receive either 30 mg of pioglitazone tablets or placebo once daily for 16 weeks in addition to their current metformin regimen. Treatment with pioglitazone tablets as add-on to metformin produced statistically significant improvements in HbA1c and FPG at endpoint compared to placebo add-on to metformin (see Table 22).
Table 22: Glycemic Parameters in a 16-Week Placebo-Controlled, Add-on to Metformin Trial Placebo
+ MetforminPioglitazone
Tablets 30 mg
+ MetforminTotal Population HbA1c (%) N=153 N=161 Baseline (mean) 9.8 9.9 Change from baseline (adjusted mean*) 0.2 -0.6 Difference from placebo + metformin (adjusted mean*) 95% Confidence Interval -0.8†
(-1.2, -0.5)Fasting Plasma Glucose (mg/dL) N=157 N=165 Baseline (mean) 260 254 Change from baseline (adjusted mean*) -5 -43 Difference from placebo + metformin (adjusted mean*) 95% Confidence Interval -38†
(-49, -26)*Adjusted for baseline, pooled center, and pooled center by treatment interaction
†p ≤ 0.05 vs. placebo + metformin
In the second trial, 827 patients were randomized to receive either 30 mg or 45 mg of pioglitazone tablets once daily for 24 weeks in addition to their current metformin regimen. The mean reduction from baseline at Week 24 in HbA1c was 0.8% for the 30 mg dose and 1.0% for the 45 mg dose (see Table 23). The mean reduction from baseline at Week 24 in FPG was 38 mg/dL for the 30 mg dose and 51 mg/dL for the 45 mg dose.
Table 23: Glycemic Parameters in a 24-Week Add-on to Metformin Study Pioglitazone
Tablets 30 mg +
MetforminPioglitazone
Tablets 45 mg +
MetforminTotal Population HbA1c (%) N=400 N=398 Baseline (mean) 9.9 9.8 Change from baseline (adjusted mean*) -0.8 -1.0 Difference from 30 mg daily pioglitazone tablets + Metformin (adjusted mean*) (95% CI) -0.2
(-0.5, 0.1)Fasting Plasma Glucose (mg/dL) N=398 N=399 Baseline (mean) 233 232 Change from baseline (adjusted mean*) -38 -51 Difference from 30 mg daily pioglitazone tablets + Metformin (adjusted mean*) (95% CI) -12†
(-21, -4)95% CI = 95% confidence interval
*Adjusted for baseline, pooled center, and pooled center by treatment interaction
†p ≤ 0.05 vs. 30 mg daily pioglitazone tablets + metformin
The therapeutic effect of pioglitazone tablets in combination with metformin was observed in patients regardless of the metformin dose.
Add-on to Insulin Trials
Two clinical trials were conducted with pioglitazone tablets in combination with insulin. Both trials included patients with type 2 diabetes on insulin, either alone or in combination with another antidiabetic agent. All other antidiabetic agents were withdrawn prior to starting study treatment. In the first trial, 566 patients were randomized to receive either 15 mg or 30 mg of pioglitazone tablets or placebo once daily for 16 weeks in addition to their insulin regimen. Treatment with pioglitazone tablets as add-on to insulin produced statistically significant improvements in HbA1c and FPG at endpoint compared to placebo add-on to insulin (see Table 24). The mean daily insulin dose at baseline in each treatment group was approximately 70 units. The majority of patients (75% overall, 86% treated with placebo, 77% treated with pioglitazone tablets 15 mg, and 61% treated with pioglitazone tablets 30 mg) had no change in their daily insulin dose from baseline to the final study visit. The mean change from baseline in daily dose of insulin (including patients with no insulin dose modifications) was -3 units in the patients treated with pioglitazone tablets 15 mg, -8 units in the patients treated with pioglitazone tablets 30 mg, and -1 unit in patients treated with placebo.
Table 24: Glycemic Parameters in a 16-Week Placebo-Controlled, Add-on to Insulin Trial Placebo
+ InsulinPioglitazone Tablets 15 mg
+ InsulinPioglitazone Tablets 30 mg
+ InsulinTotal Population HbA1c (%) N=177 N=177 N=185 Baseline (mean) 9.8 9.8 9.8 Change from baseline (adjusted mean*) -0.3 -1.0 -1.3 Difference from placebo + Insulin (adjusted mean*) 95% Confidence Interval -0.7†
(-1.0, -0.5)-1.0†
(-1.3, -0.7)Fasting Plasma Glucose (mg/dL) N=179 N=183 N=184 Baseline (mean) 221 222 229 Change from baseline (adjusted mean*) 1 -35 -48 Difference from placebo + Insulin (adjusted mean*) 95% Confidence Interval -35†
(-51, -19)-49†
(-65, -33)*Adjusted for baseline, pooled center, and pooled center by treatment interaction
†p ≤ 0.05 vs. placebo + insulinIn the second trial, 690 patients receiving a median of 60 units per day of insulin were randomized to receive either 30 mg or 45 mg of pioglitazone tablets once daily for 24 weeks in addition to their current insulin regimen. The mean reduction from baseline at Week 24 in HbA1c was 1.2% for the 30 mg dose and 1.5% for the 45 mg dose. The mean reduction from baseline at Week 24 in FPG was 32 mg/dL for the 30 mg dose and 46 mg/dL for the 45 mg dose (see Table 25). The mean daily insulin dose at baseline in both treatment groups was approximately 70 units. The majority of patients (55% overall, 58% treated with pioglitazone tablets 30 mg, and 52% treated with pioglitazone tablets 45 mg) had no change in their daily insulin dose from baseline to the final study visit. The mean change from baseline in daily dose of insulin (including patients with no insulin dose modifications) was -5 units in the patients treated with pioglitazone tablets 30 mg and -8 units in the patients treated with pioglitazone tablets 45 mg.
The therapeutic effect of pioglitazone tablets in combination with insulin was observed in patients regardless of the insulin dose.
Table 25: Glycemic Parameters in a 24-Week Add-on to Insulin Trial Pioglitazone Tablets
30 mg +
InsulinPioglitazone Tablets
45 mg +
InsulinTotal Population HbA1c (%) N=328 N=328 Baseline (mean) 9.9 9.7 Change from baseline (adjusted mean*) -1.2 -1.5 Difference from 30 mg daily pioglitazone tablets + Insulin (adjusted mean*) (95% CI) -0.3†
(-0.5, -0.1)Fasting Plasma Glucose (mg/dL) N=325 N=327 Baseline (mean) 202 199 Change from baseline (adjusted mean*) -32 -46 Difference from 30 mg daily pioglitazone tablets + Insulin (adjusted mean*) (95% CI) -14†
(-25, -3)95% CI = 95% confidence interval
*Adjusted for baseline, pooled center, and pooled center by treatment interaction
†p ≤ 0.05 vs. 30 mg daily pioglitazone tablets + insulin
-
16 HOW SUPPLIED/ STORAGE AND HANDLING
Pioglitazone tablets USP are available in 15 mg, 30 mg, and 45 mg tablets as follows:
15 mg tablet: White, round, flat face beveled edge, non-scored tablet with “W” on one side, and “15” on the other, available in:
Bottles of 30: NDC: 72606-501-01
Bottles of 90: NDC: 72606-501-02
Bottles of 500: NDC: 72606-501-03
30 mg tablet: White, round, flat face beveled edge, non-scored tablet with “W” on one side, and “30” on the other, available in:
Bottles of 30: NDC: 72606-502-01
Bottles of 90: NDC: 72606-502-02
Bottles of 500: NDC: 72606-502-03
45 mg tablet: White, round, flat face beveled edge, non-scored tablet with “W” on one side, and “45” on the other, available in:
Bottles of 30: NDC: 72606-503-01
Bottles of 90: NDC: 72606-503-02
Bottles of 500: NDC: 72606-503-03
Storage: Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Keep container tightly closed, and protect from light, moisture and humidity.
-
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling (Medication Guide).
- It is important to instruct patients to adhere to dietary instructions and to have blood glucose and glycosylated hemoglobin tested regularly. During periods of stress such as fever, trauma, infection, or surgery, medication requirements may change and patients should be reminded to seek medical advice promptly.
- Patients who experience an unusually rapid increase in weight or edema or who develop shortness of breath or other symptoms of heart failure while on pioglitazone tablets should immediately report these symptoms to a physician.
- Tell patients to promptly stop taking pioglitazone tablets and seek immediate medical advice if there is unexplained nausea, vomiting, abdominal pain, fatigue, anorexia, or dark urine as these symptoms may be due to hepatotoxicity.
- Tell patients to promptly report any sign of macroscopic hematuria or other symptoms such as dysuria or urinary urgency that develop or increase during treatment as these may be due to bladder cancer.
- Tell patients to take pioglitazone tablets once daily. Pioglitazone tablets can be taken with or without meals. If a dose is missed on one day, the dose should not be doubled the following day.
- When using combination therapy with insulin or other antidiabetic medications, the risks of hypoglycemia, its symptoms and treatment, and conditions that predispose to its development should be explained to patients and their family members.
- Inform female patients that treatment with pioglitazone tablets, like other thiazolidinediones, may result in an unintended pregnancy in some premenopausal anovulatory females due to its effect on ovulation [see Use in Specific Populations (8.3)].
Manufactured by:
CELLTRION PHARM, INC.
82, 2sandan-ro, Ochang-eup, Cheongwon-gu, Cheongju-si, Chungcheongbuk-do, 28117, Republic of Korea
Manufactured for:
CELLTRION USA, INC.
One Evertrust Plaza Suite 1207 Jersey City, New Jersey 07302
Revised: 04/2019

-
MEDICATION GUIDE
MEDICATION GUIDE
Pioglitazone (PYE-oh-GLI-ta-zone) Tablets USP
Read this Medication Guide carefully before you start taking pioglitazone tablets and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment. If you have any questions about pioglitazone tablets, ask your doctor or pharmacist.
What is the most important information I should know about pioglitazone tablets?
Pioglitazone tablets can cause serious side effects, including new or worse heart failure.
- Pioglitazone tablets can cause your body to keep extra fluid (fluid retention), which leads to swelling (edema) and weight gain. Extra body fluid can make some heart problems worse or lead to heart failure. Heart failure means your heart does not pump blood well enough.
- Do not take pioglitazone tablets if you have severe heart failure.
- If you have heart failure with symptoms (such as shortness of breath or swelling), even if these symptoms are not severe, pioglitazone tablets may not be right for you.
Call your doctor right away if you have any of the following:
- swelling or fluid retention, especially in the ankles or legs
- shortness of breath or trouble breathing, especially when you lie down
- an unusually fast increase in weight
- unusual tiredness
Pioglitazone tablets can have other serious side effects. See “What are the possible side effects of pioglitazone tablets?”
What are pioglitazone tablets?
Pioglitazone tablets are a prescription medicine used with diet and exercise to improve blood sugar (glucose) control in adults with type 2 diabetes. Pioglitazone tablets are a diabetes medicine called pioglitazone that may be taken alone or with other diabetes medicines.
It is not known if pioglitazone tablets are safe and effective in children under the age of 18. Pioglitazone is not recommended for use in children.
Pioglitazone is not for people with type 1 diabetes.
Pioglitazone is not for people with diabetic ketoacidosis (increased ketones in your blood or urine).
Who should not take pioglitazone tablets?
See “What is the most important information I should know about pioglitazone tablets?”
Do not take pioglitazone tablets if you:
- have severe heart failure.
- are allergic to any of the ingredients in pioglitazone tablets. See the end of this Medication Guide for a complete list of ingredients in pioglitazone tablets.
Talk to your doctor before taking pioglitazone tablets if you have either of these conditions.
What should I tell my doctor before taking pioglitazone tablets?
Before you take pioglitazone tablets, tell your doctor if you:
- have heart failure
- have type 1 (“juvenile”) diabetes or had diabetic ketoacidosis
- have a type of diabetic eye disease that causes swelling in the back of the eye (macular edema)
- have liver problems
- have or have had cancer of the bladder
- are pregnant or plan to become pregnant. It is not known if pioglitazone tablets can harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant about the best way to control your blood glucose levels while pregnant.
- are a premenopausal woman (before the “change of life”) who does not have periods regularly or at all. Pioglitazone tablets may increase your chance of becoming pregnant. Talk to your doctor about birth control choices while taking pioglitazone tablets. Tell your doctor right away if you become pregnant while taking pioglitazone tablets.
- are breastfeeding or plan to breastfeed. It is not known if pioglitazone passes into your milk and if it can harm your baby. Talk to your doctor about the best way to control your blood glucose levels while breastfeeding.
Tell your doctor about all the medicines you take including prescription and over the counter medicines, vitamins, and herbal supplements.
Pioglitazone tablets and some of your other medicines can affect each other. You may need to have your dose of pioglitazone tablets or certain other medicines changed.
Know the medicines you take. Keep a list of your medicines and show it to your doctor and pharmacist before you start a new medicine. They will tell you if it is okay to take pioglitazone tablets with other medicines.
How should I take pioglitazone tablets?
- Take pioglitazone tablets exactly as your doctor tells you to take it.
- Your doctor may change your dose of pioglitazone tablets. Do not change your pioglitazone tablets dose unless your doctor tells you to.
- Pioglitazone tablets may be prescribed alone or with other diabetes medicines. This will depend on how well your blood sugar is controlled.
- Take a pioglitazone tablet one time each day, with or without food.
- If you miss a dose of pioglitazone tablets, take your next dose as prescribed unless your doctor tells you differently. Do not take two doses at one time the next day.
- If you take too many pioglitazone tablets, call your doctor or go to the nearest hospital emergency room right away.
- If your body is under stress such as from a fever, infection, accident, or surgery the dose of your diabetes medicines may need to be changed. Call your doctor right away.
- Stay on your diet and exercise programs and test your blood sugar regularly while taking pioglitazone tablets.
- Your doctor should do certain blood tests before you start and while you take pioglitazone tablets.
- Your doctor should also do hemoglobin A1C testing to check how well your blood sugar is controlled with pioglitazone tablets.
- Your doctor should check your eyes regularly while you take pioglitazone tablets.
What are the possible side effects of pioglitazone tablets?
Pioglitazone tablets may cause serious side effects including:
-
See “What is the most important information I should know about pioglitazone tablets.”
- low blood sugar (hypoglycemia). This can happen if you skip meals, if you also use another medicine that lowers blood sugar, or if you have certain medical problems. Lightheadedness, dizziness, shakiness, or hunger may happen if your blood sugar is too low. Call your doctor if low blood sugar levels are a problem for you.
- liver problems. Call your doctor right away if you have:
o nausea or vomiting
o stomach pain
o unusual or unexplained tiredness
o loss of appetite
o dark urine
o yellowing of your skin or the whites of your eyes
- bladder cancer. There may be an increased chance of having bladder cancer when you take pioglitazone tablets. You should not take pioglitazone tablets if you are receiving treatment for bladder cancer. Tell your doctor right away if you have any of the following symptoms of bladder cancer:
o blood or a red color in your urine
o an increased need to urinate
o pain while you urinate
- broken bones (fractures). Usually in the hand, upper arm, or foot in women. Talk to your doctor for advice on how to keep your bones healthy.
- diabetic eye disease with swelling in the back of the eye (macular edema). Tell your doctor right away if you have any changes in your vision. Your doctor should check your eyes regularly
- release of an egg from an ovary in a woman (ovulation) leading to pregnancy. Ovulation may happen when premenopausal women who do not have regular monthly periods take pioglitazone tablets. This can increase your chance of getting pregnant
The most common side effects of pioglitazone tablets include:
o cold-like symptoms (upper respiratory tract infection)
o headache
o sinus infection
o muscle pain
o sore throat
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the side effects of pioglitazone tablets. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store pioglitazone tablets?
- Store pioglitazone tablets at 68° to 77°F (20°C to 25°C). Keep pioglitazone tablets in the original container and protect from light.
- Keep the pioglitazone tablets bottle tightly closed and keep tablets dry.
- Keep pioglitazone tablets and all medicines out of the reach of children.
General information about the safe and effective use of pioglitazone tablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use pioglitazone tablets for a condition for which it was not prescribed. Do not give pioglitazone tablets to other people, even if they have the same symptoms you have. It may harm them.
This Medication Guide summarizes the most important information about pioglitazone tablets. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about pioglitazone tablets that is written for healthcare professionals. For more information, call 1-844-837-6511.
What are the ingredients in pioglitazone tablets?
Active Ingredient: pioglitazone hydrochloride
Inactive Ingredients: lactose monohydrate, hydroxypropylcellulose, carboxymethylcellulose calcium, and magnesium stearate.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Manufactured by:
CELLTRION PHARM, INC.
82, 2sandan-ro, Ochang-eup, Cheongwon-gu, Cheongju-si, Chungcheongbuk-do, 28117, Republic of Korea
Manufactured for:
CELLTRION USA, INC.
One Evertrust Plaza Suite 1207 Jersey City, New Jersey 07302
Revised: 04/2019

-
Principal Display Panel - 15 mg Bottle Label
NDC: 72606-501-01
Pioglitazone Tablets USP
15 mg
PHARMACIST: Dispense with attached Medication Guide
Rx only 30 Tablets
-
Principal Display Panel - 15 mg Bottle Label
NDC: 72606-501-02
Pioglitazone Tablets USP
15 mg
PHARMACIST: Dispense with attached Medication Guide
Rx only 90 Tablets
-
Principal Display Panel - 15 mg Bottle Label
NDC: 72606-501-03
Pioglitazone Tablets USP
15 mg
PHARMACIST: Dispense with attached Medication Guide
Rx only 500 Tablets
-
Principal Display Panel - 30 mg Bottle Label
NDC: 72606-502-01
Pioglitazone Tablets USP
30 mg
PHARMACIST: Dispense with attached Medication Guide
Rx only 30 Tablets
-
Principal Display Panel - 30 mg Bottle Label
NDC: 72606-502-02
Pioglitazone Tablets USP
30 mg
PHARMACIST: Dispense with attached Medication Guide
Rx only 90 Tablets
-
Principal Display Panel - 30 mg Bottle Label
NDC: 72606-502-03
Pioglitazone Tablets USP
30 mg
PHARMACIST: Dispense with attached Medication Guide
Rx only 500 Tablets
-
Principal Display Panel - 45 mg Bottle Label
NDC: 72606-503-01
Pioglitazone Tablets USP
45 mg
PHARMACIST: Dispense with attached Medication Guide
Rx only 30 Tablets
-
Principal Display Panel - 45 mg Bottle Label
NDC: 72606-503-02
Pioglitazone Tablets USP
45 mg
PHARMACIST: Dispense with attached Medication Guide
Rx only 90 Tablets
-
Principal Display Panel - 45 mg Bottle Label
NDC: 72606-503-03
Pioglitazone Tablets USP
45 mg
PHARMACIST: Dispense with attached Medication Guide
Rx only 500 Tablets
-
INGREDIENTS AND APPEARANCE
PIOGLITAZONE HYDROCHLORIDE
pioglitazone hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72606-501 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PIOGLITAZONE HYDROCHLORIDE (UNII: JQT35NPK6C) (PIOGLITAZONE - UNII:X4OV71U42S) PIOGLITAZONE 15 mg Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE CALCIUM (UNII: UTY7PDF93L) HYDROXYPROPYL CELLULOSE (1200000 WAMW) (UNII: U3JF91U133) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white Score no score Shape ROUND Size 6mm Flavor Imprint Code W;15 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72606-501-01 30 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2019 2 NDC: 72606-501-02 90 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2019 3 NDC: 72606-501-03 500 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076798 10/26/2012 PIOGLITAZONE HYDROCHLORIDE
pioglitazone hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72606-502 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PIOGLITAZONE HYDROCHLORIDE (UNII: JQT35NPK6C) (PIOGLITAZONE - UNII:X4OV71U42S) PIOGLITAZONE 30 mg Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE CALCIUM (UNII: UTY7PDF93L) HYDROXYPROPYL CELLULOSE (1200000 WAMW) (UNII: U3JF91U133) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white Score no score Shape ROUND Size 8mm Flavor Imprint Code W;30 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72606-502-01 30 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2019 2 NDC: 72606-502-02 90 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2019 3 NDC: 72606-502-03 500 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076798 10/26/2012 PIOGLITAZONE HYDROCHLORIDE
pioglitazone hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72606-503 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PIOGLITAZONE HYDROCHLORIDE (UNII: JQT35NPK6C) (PIOGLITAZONE - UNII:X4OV71U42S) PIOGLITAZONE 45 mg Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE CALCIUM (UNII: UTY7PDF93L) HYDROXYPROPYL CELLULOSE (1200000 WAMW) (UNII: U3JF91U133) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white Score no score Shape ROUND Size 9mm Flavor Imprint Code W;45 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72606-503-01 30 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2019 2 NDC: 72606-503-02 90 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2019 3 NDC: 72606-503-03 500 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076798 10/26/2012 Labeler - CELLTRION USA, INC. (116587378) Registrant - CELLTRION, INC. (688836030) Establishment Name Address ID/FEI Business Operations CELLTRION PHARM, INC. 689687234 pack(72606-501, 72606-502, 72606-503) , label(72606-501, 72606-502, 72606-503) , manufacture(72606-501, 72606-502, 72606-503) Establishment Name Address ID/FEI Business Operations Cipla Ltd. 650072015 manufacture(72606-501, 72606-502, 72606-503)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.