Germ-X 842.000/842AA Antibacterial Hand Soap Fresh Scent
Moisturizing Antibacterial by
Drug Labeling and Warnings
Moisturizing Antibacterial by is a Otc medication manufactured, distributed, or labeled by UpLift Brands, LLC, Consumer Product Partners, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
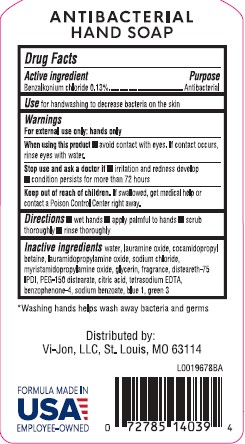
MOISTURIZING ANTIBACTERIAL- benzalkonium chloride 0.13% soap
UpLift Brands, LLC
----------
Germ-X 842.000/842AA
Antibacterial Hand Soap Fresh Scent
When using this product
- avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Stop use and ask a doctor if
- irritation and redness develop
- condition persists for more than 72 hours
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
| MOISTURIZING ANTIBACTERIAL
benzalkonium chloride 0.13% soap |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - UpLift Brands, LLC (119091527) |
| Registrant - Consumer Product Partners, LLC (119091520) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Consumer Product Partners, LLC | 119091520 | manufacture(83986-842) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Consumer Product Partners, LLC | 119091514 | manufacture(83986-842) | |
Revised: 9/2025
Document Id: 4005c71a-921d-f660-e063-6394a90a7a2c
Set id: d86230a0-54ba-4f8d-9011-12fd2efc7489
Version: 3
Effective Time: 20250930
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.