TIGAN- trimethobenzamide hydrochloride injection
Tigan by
Drug Labeling and Warnings
Tigan by is a Prescription medication manufactured, distributed, or labeled by General Injectables & Vaccines, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
Description
Chemically, trimethobenzamide HCl is N-[p-[2-(dimethylamino)ethoxy]benzyl]-3,4,5-trimethoxybenzamide monohydrochloride. It
has a molecular weight of 424.93 and the following structural formula: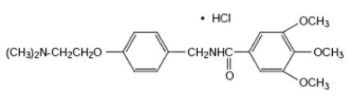
Single-Dose Vials: Each 2-mL single-dose vial contains 200 mg trimethobenzamide hydrochloride compounded with 1 mg sodium
citrate and 0.4 mg citric acid as buffers and pH adjusted to approximately 5.0 with sodium hydroxide.
Multi-Dose Vials: Each mL contains 100 mg trimethobenzamide hydrochloride compounded with 0.45% phenol as preservative, 0.5
mg sodium citrate and 0.2 mg citric acid as buffers and pH adjusted to approximately 5.0 with sodium hydroxide. -
Clinical Pharmacology
Mechanism of Action
The mechanism of action of Tigan® as determined in animals is obscure, but may involve the chemoreceptor trigger zone (CTZ), an
area in the medulla oblongata through which emetic impulses are conveyed to the vomiting center; direct impulses to the vomiting
center apparently are not similarly inhibited. In dogs pretreated with trimethobenzamide HCl, the emetic response to apomorphine is
inhibited, while little or no protection is afforded against emesis induced by intragastric copper sulfate.Pharmacokinetics
The pharmacokinetics of trimethobenzamide have been studied in healthy adult subjects. Following administration of 200 mg
(100 mg/mL) Tigan I.M. injection, the time to reach maximum plasma concentration (Tmax) was about half an hour, about 15
minutes longer for Tigan 300 mg oral capsule than an I.M. injection. A single dose of Tigan 300 mg oral capsule provided a plasma
concentration profile of trimethobenzamide similar to Tigan 200 mg I.M. The relative bioavailability of the capsule formulation
compared to the solution is 100%. The mean elimination half-life of trimethobenzamide is 7 to 9 hours. Between 30 – 50% of a single
dose in humans is excreted unchanged in the urine within 48 – 72 hours. The metabolic disposition of trimethobenzamide in humans is
not known. Specifically, it is not known if active metabolites are generated in humans.Special Populations
Age
The clearance of trimethobenzamide is not known in patients with renal impairment. However, it may be advisable to consider
reduction in the dosing of trimethobenzamide in elderly patients with renal impairment considering that a substantial amount of
excretion and elimination of trimethobenzamide occurs via the kidney and that elderly patients may have various degrees of renal
impairment. (See PRECAUTIONS: General and DOSAGE AND ADMINISTRATION).Gender
Systemic exposure to trimethobenzamide was similar between men (N=40) and women (N=28).Race
Pharmacokinetics appeared to be similar for Caucasians (N=53) and African Americans (N=12).Renal Impairment
The clearance of trimethobenzamide is not known in patients with renal impairment. However, it may be advisable to consider
reduction in the dosing of trimethobenzamide in patients with renal impairment considering that a substantial amount of
excretion and elimination of trimethobenzamide occurs via the kidney. (See PRECAUTIONS: General and DOSAGE AND
ADMINISTRATION). - Indications
- Contraindications
- Contraindications
-
Warnings
Tigan® may produce drowsiness. Patients should not operate motor vehicles or other dangerous machinery until their individual
responses have been determined.Usage in Pregnancy
Trimethobenzamide hydrochloride was studied in reproduction experiments in rats and rabbits and no teratogenicity was suggested.
The only effects observed were an increased percentage of embryonic resorptions or stillborn pups in rats administered 20 mg and
100 mg/kg and increased resorptions in rabbits receiving 100 mg/kg. In each study these adverse effects were attributed to one or
two dams. The relevance to humans is not known. Since there is no adequate experience in pregnant or lactating women who have
received this drug, safety in pregnancy or in nursing mothers has not been established.Usage with Alcohol
Concomitant use of alcohol with Tigan® may result in an adverse drug interaction. -
Precautions
During the course of acute febrile illness, encephalitides, gastroenteritis, dehydration and electrolyte imbalance, especially in
children and the elderly or debilitated, CNS reactions such as opisthotonos, convulsions, coma and extrapyramidal symptoms have
been reported with and without use of Tigan® (trimethobenzamide hydrochloride) or other antiemetic agents. In such disorders
caution should be exercised in administering Tigan®, particularly to patients who have recently received other CNS-acting agents
(phenothiazines, barbiturates, belladonna derivatives). Primary emphasis should be directed toward the restoration of body fluids and
electrolyte balance, the relief of fever and relief of the causative disease process. Overhydration should be avoided since it may result
in cerebral edema.
The antiemetic effects of Tigan® may render diagnosis more difficult in such conditions as appendicitis and obscure signs of toxicity
due to overdosage of other drugs.General
Adjustment of Dose in Renal Failure
A substantial route of elimination of unchanged trimethobenzamide is via the kidney. Dosage adjustment should be considered in
patients with reduced renal function including some elderly patients. (See CLINICAL PHARMACOLOGY and DOSAGE AND
ADMINISTRATION).Geriatric Use
Clinical studies of trimethobenzamide hydrochloride did not include sufficient numbers of patients aged 65 and over to determine
whether they respond differently from younger patients. Although there are studies reported in the literature that included elderly
patients greater than 65 years old with younger patients, it is not known if there are differences in efficacy or safety parameters for elderly and
non-elderly patients treated with trimethobenzamide. In general, dose selection for an elderly patient should be cautious, usually
starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of
concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients
with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in
dose selection, and it may be useful to monitor renal function. (See CLINICAL PHARMACOLOGY and DOSAGE AND
ADMINISTRATION). -
Adverse Reactions
There have been reports of hypersensitivity reactions and Parkinson-like symptoms. There have been instances of hypotension
reported following parenteral administration to surgical patients. There have been reports of blood dyscrasias, blurring of vision,
coma, convulsions, depression of mood, diarrhea, disorientation, dizziness, drowsiness, headache, jaundice, muscle cramps
and opisthotonos. If these occur, the administration of the drug should be discontinued. Allergic-type skin reactions have been
observed; therefore, the drug should be discontinued at the first sign of sensitization. While these symptoms will usually disappear
spontaneously, symptomatic treatment may be indicated in some cases.For medical advice about adverse reactions contact your medical professional. To report SUSPECTED ADVERSE REACTIONS,
contact JHP at 1-866-923-2547 or MEDWATCH at 1-800-FDA-1088 (1-800-332-1088) or http://www.fda.gov/medwatch/. -
Dosage and Administration
(See WARNINGS and PRECAUTIONS.)
Dosage should be adjusted according to the indication for therapy, severity of symptoms and the response of the patient.Geriatric Patients
Dose adjustment such as reducing the total dose administered at each dosing or increasing the dosing interval should be considered
in elderly patients with renal impairment (creatinine clearance £ 70 mL/min/1.73m2). Final dose adjustment should be based upon
integration of clinical efficacy and safety considerations. (See CLINICAL PHARMACOLOGY and PRECAUTIONS).Patients with Renal Impairment
In subjects with renal impairment (creatinine clearance £ 70 mL/min/1.73m2), dose adjustment such as reducing the total dose
administered at each dosing or increasing the dosing interval should be considered. (See CLINICAL PHARMACOLOGY and
DOSAGE AND ADMINISTRATION).INJECTABLE, 100 mg/mL (Not for use in pediatric patients)
Usual Adult Dosage
2 mL (200 mg) t.i.d. or q.i.d. intramuscularly.NOTE: The injectable form is intended for intramuscular administration only; it is not recommended for intravenous use.
Intramuscular administration may cause pain, stinging, burning, redness and swelling at the site of injection. Such effects may be
minimized by deep injection into the upper outer quadrant of the gluteal region, and by avoiding the escape of solution along the route. - Storage
-
How Supplied
Tigan® (trimethobenzamide hydrochloride) is available as follows:
NDC: 42023-119-25 100 mg/mL in 2 mL Single-Dose Vials, Pack of 25
NDC: 42023-118-01 100 mg/mL in 20 mL Multi-Dose Vials, Pack of 1
Rx Only
Manufacturered by:
Par Pharmaceutical
Chestnut Ridge, NY
R04/16
3000358F
OS118J-01-90-01
- Sample Outer Label
-
INGREDIENTS AND APPEARANCE
TIGAN
trimethobenzamide hydrochloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 52584-119(NDC:42023-119) Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Trimethobenzamide Hydrochloride (UNII: WDQ5P1SX7Q) (Trimethobenzamide - UNII:W2X096QY97) Trimethobenzamide Hydrochloride 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CITRATE (UNII: 1Q73Q2JULR) 0.5 mg in 1 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 0.2 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52584-119-25 1 in 1 BAG 08/01/2010 1 2 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA017530 08/01/2010 Labeler - General Injectables & Vaccines, Inc (108250663)
Trademark Results [Tigan]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TIGAN 72064174 0684353 Live/Registered |
HOFFMANN-LA ROCHE INC. 1958-12-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
