HEVERT CALMVALERA COMP.- black cohosh, anamirta cocculus seed, cypripedium parvifolum root, strychnos ignatii seed, passiflora incarnata flowering top, platinum, valerian, and zinc valerate dihydrate injection
Hevert Calmvalera comp. by
Drug Labeling and Warnings
Hevert Calmvalera comp. by is a Homeopathic medication manufactured, distributed, or labeled by Hevert Pharmaceuticals LLC, Hevert Arzneimittel GmbH & Co. KG, Solupharm Pharmazeutische Erzeugnisse GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
SPL UNCLASSIFIED SECTION
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Hevert® Calmvalera™ comp. safely and effectively.
See full prescribing information for Hevert® Calmvalera™ comp.
Hevert® Calmvalera™ comp. solution for injection, for intravenous, intramuscular and subcutaneous administration.
Rx Use Only
------------- INDICATIONS AND USAGE -------------
Hevert® Calmvalera™ comp. is a homeopathic drug indicated for the treatment of nervous disorders such as restlessness and sleep disorders, mild depressive states, and mental exhaustion. (1)
--------- DOSAGE AND ADMINISTRATION ---------
- Standard Dosage:
Adults and children 12 years and older:
1 mL to 2 mL 1 to 3 times per 7 days. - Acute Dosage:
Adults and children 12 years and older:
1 mL to 2 mL up to 3 times daily.
-------- DOSAGE FORMS AND STRENGTHS -------- - One ampule containing 2 mL solution for injection, containing the active ingredients in the strengths listed under Description. (3)
- Hevert® Calmvalera™ comp. is contraindicated in patients with known hypersensitivity to lady's slipper or orchids, or to any of its ingredients. (4)
- Keep out of reach of children. (5)
- No adverse events have been reported with causal relationship to Hevert® Calmvalera™ comp. (6.1)
- To report SUSPECTED ADVERSE REACTIONS, contact Hevert Pharmaceuticals at 1-855-387-6466 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
- None known. (7)
- No studies have been conducted with Hevert® Calmvalera™ comp. on pregnant or lactating women, in patients under 18 years of age, or elderly. (8)
FULL PRESCRIBING INFORMATION: CONTENTS*
1 Indications and Usage
2 Dosage and Administration
2.1 General Instructions
2.2 Standard Dosage
2.3 Acute Dosage
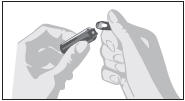
2.4 Instruction for Opening Glass Ampule
3 Dosage Forms and Strength
4 Contraindications
5 Warnings and Precautions
6 Adverse Reactions
6.1 Post-marketing Experience
7 Drug Interactions
8 Use in specific Populations
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 Overdosage
11 Description
11.1 Ingredients
11.2 Pharmaceutical Form
11.3 Route of Administration
15 References
16 How Supplied / Storage and Handling
16.1 Dosage Forms and Package Sizes
16.2 Storage
* Sections or subsections omitted from the full prescribing information are not listed. - Standard Dosage:
- 1 Indications and Usage
-
2 Dosage and Administration
2.1 General Instructions
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- Draw up required dose into syringe.
- Discard any unused ampule contents. Do not reuse ampule.
- 3 Dosage Forms and Strengths
- 4 Contraindications
- 5 Warnings and Precautions
- 6 Adverse Reactions
- 7 Drug Interactions
-
8 Use in specific Populations
8.1 Pregnancy
8.1.1 Teratogenic effects
Pregnancy Category C
Animal reproduction studies have not been performed with Hevert® Calmvalera™ comp. or any of its ingredients. There are not adequate and well-controlled studies in pregnant women. Hevert® Calmvalera™ comp. should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
8.3 Nursing Mothers
It is not known whether Hevert® Calmvalera™ comp. is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Hevert® Calmvalera™ comp. is administered to a nursing woman.
- 10 Overdosage
-
11 Description
11.1 Ingredients
Each 2 mL solution for injection ampule contains:
Active Ingredients: Ingredient name Potency Quantity Final dilution Cimicifuga racemosa 4X 5 % 5.5X Cocculus indicus 4X 5 % 5.5X Cypripedium pubescens 4X 5 % 5.5X Ignatia amara 6X 5 % 7.5X Passiflora incarnata 4X 5 % 5.5X Platinum metallicum 10X 5 % 11.5X Valeriana officinalis 4X 5 % 5.5X Zincum valerianicum 8X 5 % 9.5X Inactive ingredients:
Sodium chloride 0.015 g
- 15 References
-
16 How Supplied/Storage and Handling
16.1 Dosage Forms and Package Sizes
- Hevert® Calmvalera™ comp. ampules are supplied as 1 ampule of 2 mL solution for injection in packs of 10 ampules
- NDC: 54532-0036-4
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 2.0 mL Ampule Carton
NDC: 54532-0036-4
HEVERT®
CALMVALERA™
COMP.
HomeopathicSolution for Injection
Rx only
IV IM SC
10 sterile 2.0 mL ampules
hEVERT
-
INGREDIENTS AND APPEARANCE
HEVERT CALMVALERA COMP.
black cohosh, anamirta cocculus seed, cypripedium parvifolum root, strychnos ignatii seed, passiflora incarnata flowering top, platinum, valerian, and zinc valerate dihydrate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 54532-0036 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BLACK COHOSH (UNII: K73E24S6X9) (BLACK COHOSH - UNII:K73E24S6X9) BLACK COHOSH 4 [hp_X] in 2 mL ANAMIRTA COCCULUS SEED (UNII: 810258W28U) (ANAMIRTA COCCULUS SEED - UNII:810258W28U) ANAMIRTA COCCULUS SEED 4 [hp_X] in 2 mL CYPRIPEDIUM PARVIFLORUM VAR. PUBESCENS ROOT (UNII: 21Y9GZ1LZA) (CYPRIPEDIUM PARVIFLORUM VAR. PUBESCENS ROOT - UNII:21Y9GZ1LZA) CYPRIPEDIUM PARVIFLORUM VAR. PUBESCENS ROOT 4 [hp_X] in 2 mL STRYCHNOS IGNATII SEED (UNII: 1NM3M2487K) (STRYCHNOS IGNATII SEED - UNII:1NM3M2487K) STRYCHNOS IGNATII SEED 6 [hp_X] in 2 mL PASSIFLORA INCARNATA FLOWERING TOP (UNII: CLF5YFS11O) (PASSIFLORA INCARNATA FLOWERING TOP - UNII:CLF5YFS11O) PASSIFLORA INCARNATA FLOWERING TOP 4 [hp_X] in 2 mL PLATINUM (UNII: 49DFR088MY) (PLATINUM - UNII:49DFR088MY) PLATINUM 10 [hp_X] in 2 mL VALERIAN (UNII: JWF5YAW3QW) (VALERIAN - UNII:JWF5YAW3QW) VALERIAN 4 [hp_X] in 2 mL ZINC VALERATE DIHYDRATE (UNII: MN0RX54EQA) (VALERIC ACID - UNII:GZK92PJM7B) ZINC VALERATE DIHYDRATE 8 [hp_X] in 2 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54532-0036-4 10 in 1 CARTON 08/01/2016 1 2 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 08/01/2016 Labeler - Hevert Pharmaceuticals LLC (078647622) Registrant - Hevert Arzneimittel GmbH & Co. KG (318100617) Establishment Name Address ID/FEI Business Operations Hevert Arzneimittel GmbH & Co. KG 318100617 API MANUFACTURE(54532-0036) Establishment Name Address ID/FEI Business Operations Solupharm Pharmazeutische Erzeugnisse GmbH 316875129 MANUFACTURE(54532-0036)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.