Povi-One by Elevate Oral Care Pove-One
Povi-One by
Drug Labeling and Warnings
Povi-One by is a Otc medication manufactured, distributed, or labeled by Elevate Oral Care. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
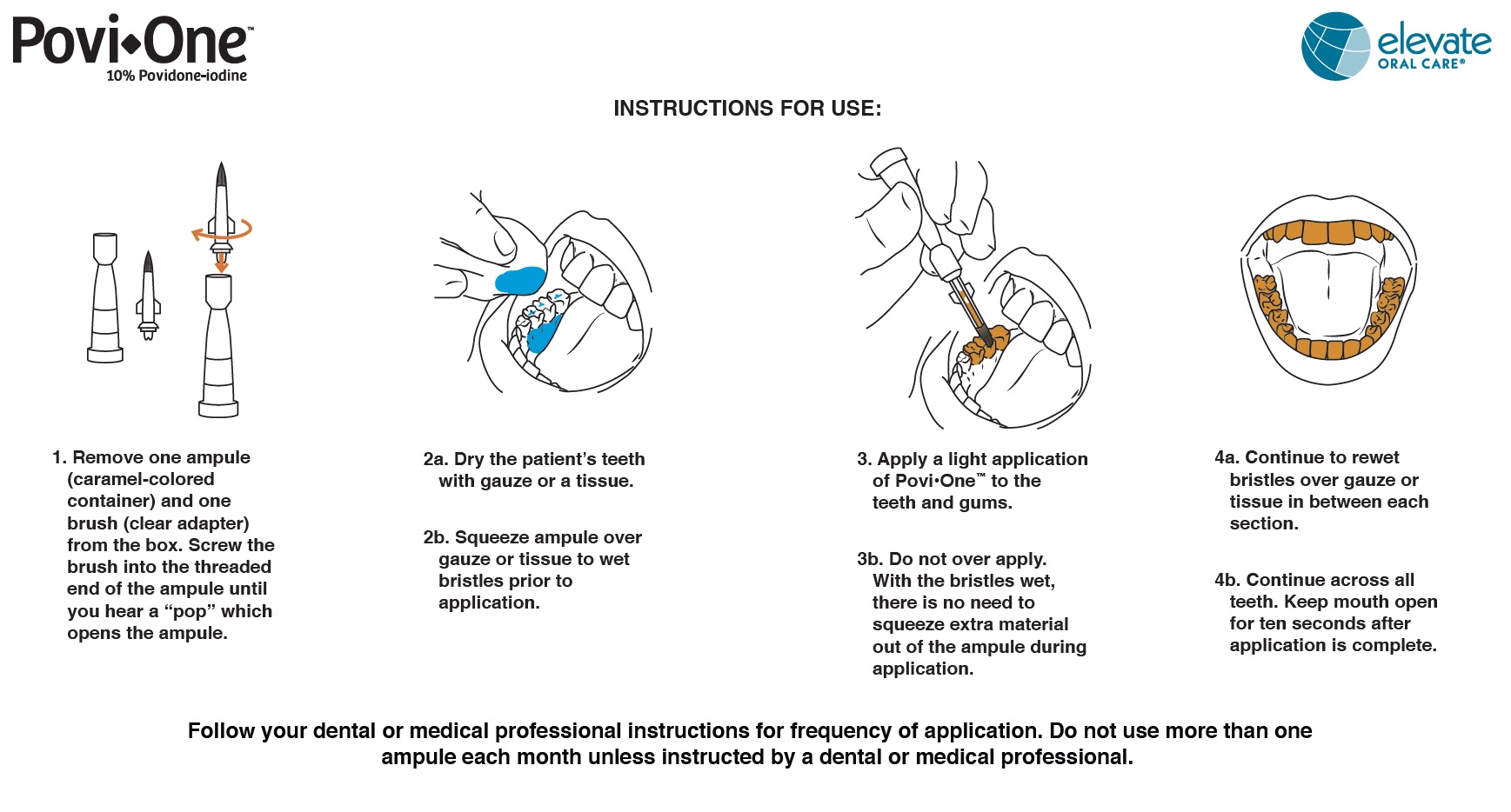
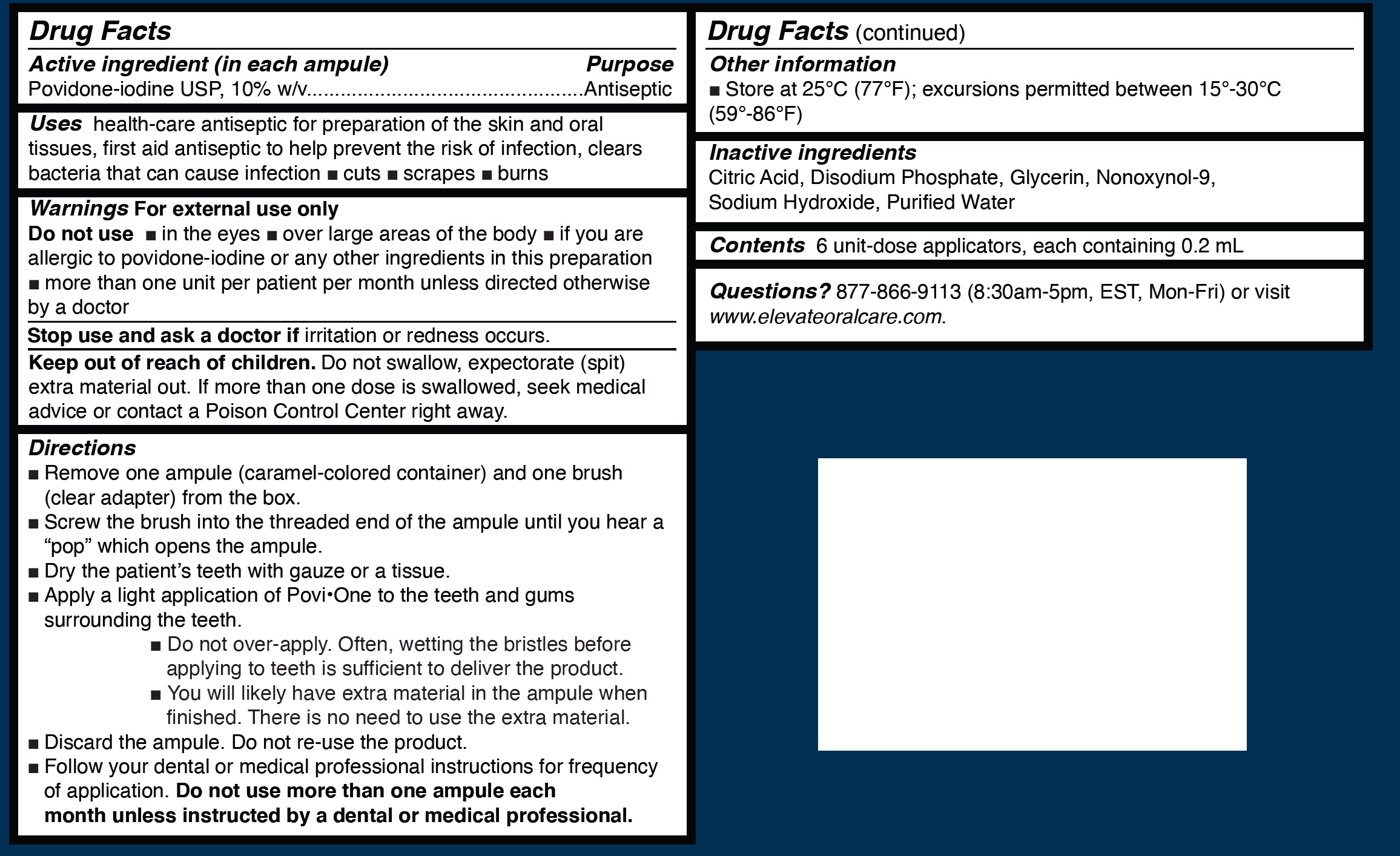
POVI-ONE- povidone-iodine 10% topical liquid
Elevate Oral Care
----------
Pove-One
| POVI-ONE
povidone-iodine 10% topical liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Elevate Oral Care (002863526) |
| Registrant - Elevate Oral Care (002863526) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Elevate Oral Care | 002863526 | relabel(57511-0610) , repack(57511-0610) | |
Revised: 5/2025
Document Id: 347a4057-37fa-f5f2-e063-6294a90a423e
Set id: d8ca1d1f-f945-480b-e053-2a95a90a096e
Version: 5
Effective Time: 20250506
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.