Nystatin by Macleods Pharmaceuticals Limited / OXALIS LABS NYSTATIN cream
Nystatin by
Drug Labeling and Warnings
Nystatin by is a Prescription medication manufactured, distributed, or labeled by Macleods Pharmaceuticals Limited, OXALIS LABS. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Nystatin is a polyene antifungal antibiotic obtained from Streptomyces nursei.
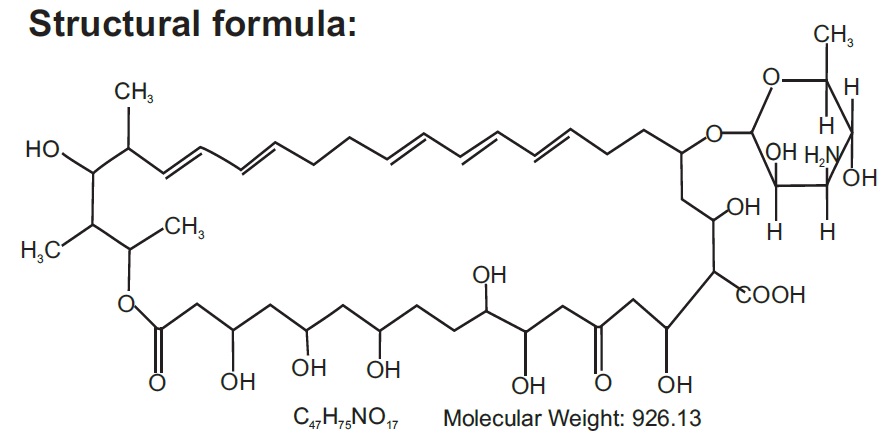
Nystatin cream is for dermatologic use.
Nystatin cream for topical use, contains 100,000 USP Nystatin Units in an aqueous cream base containing aluminium hydroxide, ceteareth-15, polyethylene glycol monostearate, glycerol monostearate, propylene glycol, purified water, simethicone emulsion, non-crystallizing sorbitol solution, titanium dioxide, white petrolatum with methyl paraben and propyl paraben as preservatives. -
CLINICAL PHARMACOLOGY
Pharmacokinetics
Nystatin is not absorbed from intact skin or mucous membrane.
Microbiology
Nystatin is an antibiotic which is both fungistatic and fungicidal in vitro against a wide variety of yeasts and yeast-like fungi, including Candida albicans, C. parapsilosis, C. tropicalis, C. guilliermondi, C. pseudotropicalis, C. krusei, Torulopsis glabrata, Tricophyton rubrum, T. mentagrophytes. Nystatin acts by binding to sterols in the cell membrane of susceptible species resulting in a change in membrane permeability and the subsequent leakage of intracellular components. On repeated subculturing with increasing levels of nystatin, Candida albicans does not develop resistance to nystatin. Generally, resistance to nystatin does not develop during therapy. However, other species of Candida (C. tropicalis, C. guilliermondi, C. krusei, and C. stellatoides) become quite resistant on treatment with Nystatin and simultaneously become cross resistant to amphotericin as well. This resistance is lost when the antibiotic is removed. Nystatin exhibits no appreciable activity against bacteria, protozoa, or viruses. - INDICATIONS & USAGE
- CONTRAINDICATIONS
-
PRECAUTIONS
GENERAL PRECAUTIONS
Nystatin cream should not be used for the treatment of systemic, oral, intravaginal or ophthalmic infections.
If irritation or sensitization develops, treatment should be discontinued and appropriate measures taken as indicated. It is recommended that KOH smears, cultures, or other diagnostic methods be used to confirm the diagnosis of cutaneous or mucocutaneous candidiasis and to rule out infection caused by other pathogens.
INFORMATION FOR PATIENTS
Patients using this medication should receive the following information and instructions:
- The patient should be instructed to use this medication as directed (including the replacement of missed doses). This medication is not for any disorder other than that for which it is prescribed.
- Even if symptomatic relief occurs within the first few days of treatment, the patient should be advised not to interrupt or discontinue therapy until the prescribed course of treatment is completed.
If symptoms of irritation develop, the patient should be advised to notify the physician promptly.
LABORATORY TESTS
If there is a lack of therapeutic response, KOH smears, cultures, or other diagnostic methods should be repeated.
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
No long-term animal studies have been performed to evaluate the carcinogenic potential of nystatin. No studies have been performed to determine the mutagenicity of nystatin or the effects on male or female fertility.
PREGNANCY
Teratogenic Effects
Animal reproduction studies have not been conducted with any nystatin cream. It also is not known whether this cream can cause fetal harm when used by a pregnant woman or can affect reproductive capacity. Nystatin cream should be prescribed for a pregnant woman only if the potential benefit to the mother outweighs the potential risk to the fetus.NURSING MOTHERS
It is not known whether nystatin is excreted in human milk. Caution should be exercised when nystatin is prescribed for a nursing woman.
PEDIATRIC USE
Safety and effectiveness have been established in the pediatric population from birth to 16 years.
(See DOSAGE AND ADMINISTRATION.)
GERIATRIC USE
Clinical studies with nystatin cream did not include sufficient numbers of subjects aged 65 years and older to determine whether they respond differently than younger subjects. Other reported clinical experience has not identified differences in responses between elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
- ADVERSE REACTIONS
- DOSAGE & ADMINISTRATION
-
HOW SUPPLIED
Nystatin Cream USP is a yellow to light green cream. It is supplied as:
15 gram Tube NDC: 33342-469-15
30 gram Tube NDC: 33342-469-30Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature]. Avoid freezing.
Manufactured for:
Macleods Pharma USA, Inc.Princeton, NJ 08540
Manufacturer:
Macleods Pharmaceuticals Limited
At Oxalis Labs
Baddi, Himachal Pradesh, INDIARev. 08/2021
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Nystatin Cream USP, 100000 units per gram
Pack Count: 15 gms Tube
NDC: 33342-469-15

Nystatin Cream USP, 100000 units/gram
Pack Count: 15 gms carton
NDC: 33342-469-15
Nystatin Cream USP, 100000 units per gram
Pack Count: 30 gms Tube
NDC: 33342-469-30
Nystatin Cream USP, 100000 units/gram
Pack Count: 30 gms carton
NDC: 33342-469-30
-
INGREDIENTS AND APPEARANCE
NYSTATIN
nystatin creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 33342-469 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NYSTATIN (UNII: BDF1O1C72E) (NYSTATIN - UNII:BDF1O1C72E) NYSTATIN 100000 in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) CETEARETH-15 (UNII: 867H4YOZ8Z) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) METHYLPARABEN (UNII: A2I8C7HI9T) SORBITOL (UNII: 506T60A25R) Product Characteristics Color YELLOW (Yellow to light green) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 33342-469-15 1 in 1 CARTON 08/25/2021 1 15 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 33342-469-30 1 in 1 CARTON 08/25/2021 2 30 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA213566 08/25/2021 Labeler - Macleods Pharmaceuticals Limited (862128535) Establishment Name Address ID/FEI Business Operations OXALIS LABS 860120472 ANALYSIS(33342-469) , LABEL(33342-469) , MANUFACTURE(33342-469) , PACK(33342-469)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.