Floriva Plus by BonGeo Pharmaceuticals, Inc. Floriva Plus™
Floriva Plus by
Drug Labeling and Warnings
Floriva Plus by is a Prescription medication manufactured, distributed, or labeled by BonGeo Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
FLORIVA PLUS- vitamin a acetate, ascorbic acid, cholecalciferol, .alpha.-tocopherol, thiamine hydrochloride, riboflavin 5-phosphate sodium, niacinamide, pyridoxine hydrochloride, levomefolate glucosamine, cyanocobalamin, biotin, pantothenic acid, and sodium fluoride solution/ drops
BonGeo Pharmaceuticals, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Floriva Plus™
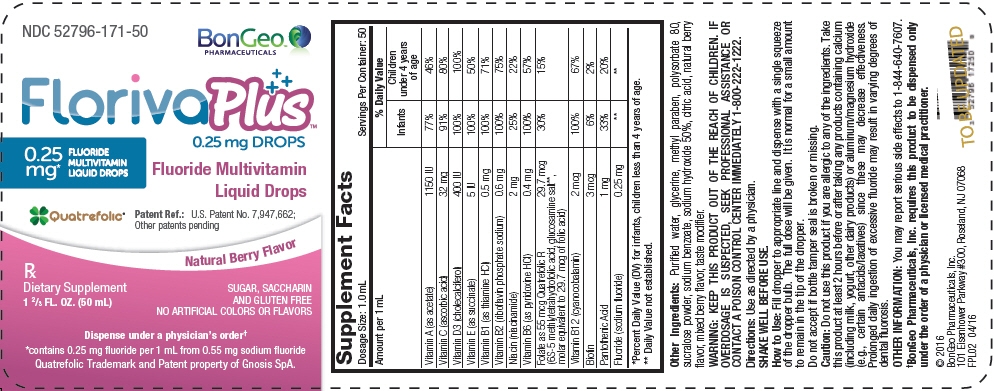
| Supplement Facts | |||
|---|---|---|---|
| Dosage Size: 1.0 mL | Servings Per Container: 50 | ||
| Amount per 1 mL | % Daily Value | ||
| Infants | Children under 4 years of age | ||
| *Percent Daily Value (DV) for infants, children less than 4 years of age. | |||
|
|
|||
| Vitamin A (as acetate) | 1150 IU | 77% | 46% |
| Vitamin C (ascorbic acid) | 32 mg | 91% | 80% |
| Vitamin D3 (cholecalciferol) | 400 IU | 100% | 100% |
| Vitamin E (as succinate) | 5 IU | 100% | 50% |
| Vitamin B1 (as thiamine HCl) | 0.5 mg | 100% | 71% |
| Vitamin B2 (riboflavin phosphate sodium) | 0.6 mg | 100% | 75% |
| Niacin (niacinamide) | 2 mg | 25% | 22% |
| Vitamin B6 (as pyridoxine HCl) | 0.4 mg | 100% | 57% |
| Folate as 55 mcg Quatrefolic R | 29.7 mcg | 30% | 15% |
| (6S-5 methlytetrahydrofolic acid, glucosamine salt*, molar equivalent to 29.7 mcg of folic acid) | |||
| Vitamin B12 (cyanocobalamin) | 2 mcg | 100% | 67% |
| Biotin | 3 mcg | 6% | 2% |
| Pantothenic Acid | 1 mg | 33% | 20% |
| Fluoride (sodium fluoride) | 0.25 mg | * | * |
Other Ingredients: Purified water, glycerine, methyl paraben, polysorbate 80, sucralose powder, sodium benzoate, sodium hydroxide 50%, citric acid, natural berry flavor, mixed berry flavor, taste modifier.
WARNING
KEEP THIS PRODUCT OUT OF THE REACH OF CHILDREN. IF OVERDOSAGE IS SUSPECTED, SEEK PROFESSIONAL ASSISTANCE OR CONTACT A POISON CONTROL CENTER IMMEDIATELY 1-800-222-1222.
How to Use
Fill dropper to appropriate line and dispense with a single squeeze of the dropper bulb. The full dose will be given. It is normal for a small amount to remain in the tip of the dropper.
Do not accept if bottle tamper seal is broken or missing.
Caution
Do not use this product if you are allergic to any of the ingredients. Take this product at least 2 hours before or after taking any products containing calcium (including milk, yogurt, other dairy products) or aluminum/magnesium hydroxide (e.g., certain antacids/laxatives) since these may decrease effectiveness. Prolonged daily ingestion of excessive fluoride may result in varying degrees of dental fluorosis.
OTHER INFORMATION
You may report serious side effects to 1-844-640-7607.
†BonGeo Pharmaceuticals, Inc. requires this product to be dispensed only under the order of a physician or licensed medical practitioner.
PRINCIPAL DISPLAY PANEL - 50 mL Container Label
NDC: 52796-171-50
BonGeo™
PHARMACEUTICALS
FlorivaPlus™
0.25 mg DROPS
0.25
mg*
FLUORIDE
MULTIVITAMIN
LIQUID DROPS
Fluoride Multivitamin
Liquid Drops
Quatrefolic®
Patent Ref.: U.S. Patent No. 7,947,662;
Other patents pending
Natural Berry Flavor
Rx
Dietary Supplement
1 ⅔ FL. OZ. (50 mL)
SUGAR, SACCHARIN
AND GLUTEN FREE
NO ARTIFICIAL COLORS OR FLAVORS
Dispense under a physician's order†
*contains 0.25 mg fluoride per 1 mL from 0.55 mg sodium fluoride
Quatrefolic Trademark and Patent property of Gnosis SpA.
| FLORIVA PLUS
vitamin a acetate, ascorbic acid, cholecalciferol, .alpha.-tocopherol, thiamine hydrochloride, riboflavin 5-phosphate sodium, niacinamide, pyridoxine hydrochloride, levomefolate glucosamine, cyanocobalamin, biotin, pantothenic acid, and sodium fluoride solution/ drops |
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Labeler - BonGeo Pharmaceuticals, Inc. (964822022) |