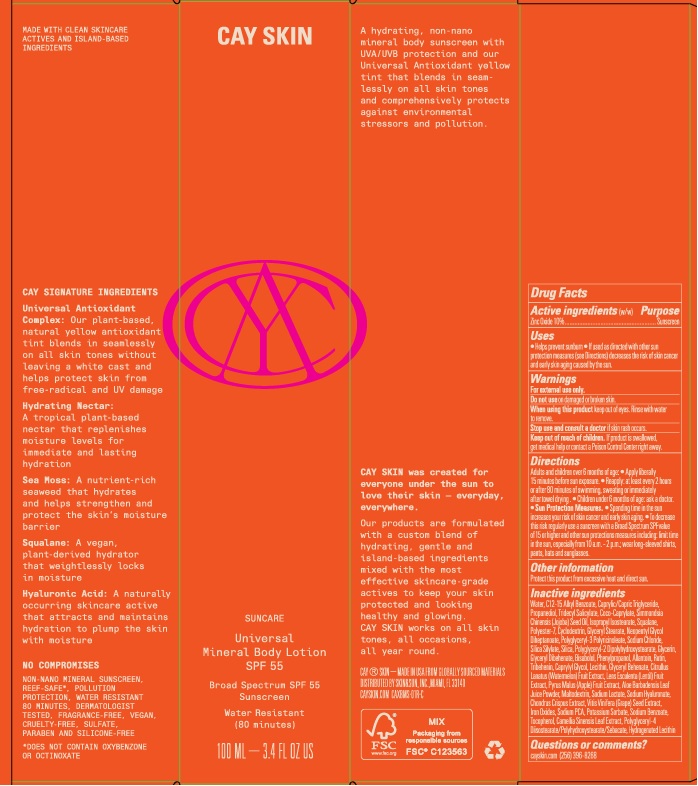
Cay Skin Universal Mineral Body Lotion SPF 55
Cay Skin Universal Mineral Body SPF 55 by
Drug Labeling and Warnings
Cay Skin Universal Mineral Body SPF 55 by is a Otc medication manufactured, distributed, or labeled by Cay Skin, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CAY SKIN UNIVERSAL MINERAL BODY SPF 55- zinc oxide lotion
Cay Skin, Inc.
----------
Cay Skin Universal Mineral Body Lotion SPF 55
Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions) decreases the risk of skin cancer and early skin aging caused by the sun.
Directions
Adults and children over 6 months of age:
- Apply liberally 15 minutes before sun exposure.
- Reapply: at least every 2 hours or after 80 minutes of swimming, sweating or immediately after towel drying.
- Children under 6 months of age: ask a doctor.
- Sun Protection Measures.
- Spending time in the sun increases your risk of skin cancer and early skin aging.
- To decrease this risk regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10 a.m. - 2 p.m.; wear long-sleeved shirts, pants, hats and sunglasses.
Inactive ingredients
Water, C12-15 Alkyl Benzoate, Caprylic/Capric Triglyceride, Propanediol, Tridecyl Salicylate, Coco-Caprylate, Simmondsia Chinensis (Jojoba) Seed Oil, Isopropyl Isostearate, Squalane, Polyester-7, Cyclodextrin, Glyceryl Stearate, Neopentyl Glycol Diheptanoate, Polyglyceryl-3 Polyricinoleate, Sodium Chloride, Siica Silylate, Siica, Polyglyceryl-2 Dpolyhydroxystearate, Glycerin, Glyceryl Dibehenate, Bisabolol, Phenylpropanol, Allantoin, Rutin, Tribehenin, Caprylyl Glycol, Lecithin, Glyceryl Behenate, Citrullus Lanatus (Watermelon) Fruit Extract, Lens Esculenta (Lentil) Fruit Extract, Pyrus Malus (Apple) Fruit Extract, Aloe Barbadensis Leaf Juice Powder, Maltodextrin, Sodium Lactate, Sodium Hyaluronate, Chondrus Crispus Extract, Vrtis Vinifera (Grape) Seed Extract, Iron Oxides, Sodium PCA, Potassium Sorbate, Sodium Benzoate, Tocopherol, Camellia Sinensis Leaf Extract, Polyglyceryl-4 Diisostearate/Polyhydroxystearate/Sebacate, Hydrogenated Lecithin
| CAY SKIN UNIVERSAL MINERAL BODY SPF 55
zinc oxide lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Cay Skin, Inc. (118574309) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.