RECONCILE- fluoxetine hydrochloride tablet, chewable
RECONCILE by
Drug Labeling and Warnings
RECONCILE by is a Animal medication manufactured, distributed, or labeled by Pegasus Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Principal Display Panel
- Indication:
- Dosage and Administration:
- Storage:
- WARNINGS:
-
Additional Information:
For complete product information, see attached package insert. For technical assistance or to report an adverse drug experience, call 1-800-874-9764. Additional information available at reconcile.com.
Manufactured by Pegasus Laboratories, Inc., Employee-Owned, Pensacola, FL 32514. Manufactured in the USA.
10-2017
PRNTM is a trademark and Reconcile® is a registered trademark of Pegasus Laboratories, Inc.
-
CLIENT INFORMATION
Your veterinarian has chosen to prescribe RECONCILE chewable tablets along with a simple training plan (a behavioral modification plan) to treat the separation anxiety that affects your dog. Please read this leaflet, which describes the use of RECONCILE chewable tablets. If you have any questions about this information, please consult your veterinarian. Additional information can be found at www.reconcile.com.
What are RECONCILE chewable tablets?
RECONCILE is a chewable, flavored tablet that you give to your dog once a day to treat separation anxiety. It is administered in conjunction with a simple training plan. RECONCILE chewable tablets are for use in dogs and puppies 6 months of age or older, and 8.8 pounds (4.0 kilograms) or greater.
What is Separation Anxiety?
Separation anxiety is a disease in which affected dogs may exhibit certain problematic behaviors when left alone. Dogs are social animals, and naturally become bonded to family members in their household. When separated from these people, certain dogs may experience distress and engage in unacceptable behaviors as a result of the anxiety of separation. The more common behaviors associated with separation anxiety include destruction of household items, barking and/or whining, soiling or urinating in the house, heavy drooling and attempting to escape. RECONCILE chewable tablets, when administered in conjunction with a simple training plan that you undertake at home, have been shown to reduce these detrimental behaviors exhibited by your dog.
What is a Simple Training Plan (Behavior Modification Plan), and why does my dog need one?
Your veterinarian will instruct you on how to incorporate simple training techniques, referred to as “behavior modification.” The combined use of these techniques with RECONCILE chewable tablets has been proven to work faster and better than training alone for the management of separation anxiety.
The simple training plan generally consists of several activities, such as:
- Adapting your dog to your coming and going behavior.
- Eaching your dog to sit and stay and, over time, to be content in your absence.
- Resisting the rewarding of inappropriate attention-seeking behaviors.
- Leaving your dog for short periods, and gradually increasing the time that the dog is left alone.
What does my veterinarian need to know about my dog before I give RECONCILE chewable tablets?
Your veterinarian is your dog’s healthcare expert and can make the best recommendation for medications for your dog. Key points of your visit may include the following:
- A thorough physical examination that may include laboratory analysis of blood and/or urine.
- A discussion of your dog's complete health history, training history and household environment.
- A discussion of any medications that you are giving now, or have given over the last several months to your dog, including over-the-counter and herbal supplements.
How should I give RECONCILE chewable tablets to my dog?
RECONCILE is a chewable flavored tablet that is readily consumed by most dogs. If your dog does not readily accept the RECONCILE chewable tablet, it may be offered in food or administered like other tablet medications. Follow your veterinarian’s directions regarding how much medication to administer.
If a dose is missed, the next scheduled dose should be given as prescribed. Do not increase or double the dose.
If more than the prescribed amount of RECONCILE chewable tablets are given, contact your veterinarian, who is the healthcare expert for your dog.
What can I expect from this therapy?
Some dogs may show improvement within 1-2 weeks of starting treatment with RECONCILE chewable tablets. Others may take as long as 8 weeks to show improvement. Your veterinarian will monitor the response to RECONCILE chewable tablets and the training plan. If no improvement is noted within 8 weeks, your veterinarian will discuss additional treatment plans for your dog.
RECONCILE chewable tablets work by making your dog more receptive to your training program. Although some dogs may appear calmer while on RECONCILE chewable tablets, it does not act as a sedative.
What side effects might occur while my dog is taking RECONCILE chewable tablets?
As with all medications, side effects may occur. Your veterinarian can best describe these for you and discuss what to do if you observe any unexpected effects or unusual behavior in your dog. The most serious side effect is seizures (convulsions), which in rare and severe cases can result in death. Based on clinical field studies, some animals may appear more calm or lethargic. Additional side effects that may be observed include: decreased appetite, vomiting, shaking or shivering, diarrhea, restlessness, excessive vocalization or whining, aggression, ear infec-tions, disorientation, incoordination, constipation, excessive salivation and weight loss. Since the introduction of RECONCILE, additional side effects reported are anxiety, dilated pupils, panting and confusion.
Can other medications be given while my pet is taking RECONCILE chewable tablets?
Yes, RECONCILE chewable tablets have been given safely with a wide variety of routinely administered products and medications, including vaccines, antibiotics, anti-inflammatories and products used for the control of fleas, intestinal worms and heartworms. There are a few products and medications that you should not give to your dog before, during or after treatment with RECONCILE chewable tablets because together, they can cause serious side effects. Your veterinarian should be made aware of all products, including over-the-counter and herbal supplements, that you intend to administer to your dog.
What else should I know about RECONCILE chewable tablets?
RECONCILE chewable tablets are not for use in humans. As with all medications, keep RECONCILE chewable tablets out of reach of children.
How Supplied:
RECONCILE is supplied in 8mg, 16mg, 32mg and 64mg strengths; as 30 tablets per bottle, with a child-resistant cap.
NADA #141-272, Approved by FDA.
Close the lid tightly between uses and keep the desiccant in the bottle until the medication is finished.
Store at 68-77°F (20-25°C). Temporary periods of time outside of this range between 59-86°F (15-30°C) are permitted.
If you have questions regarding the use of this product, consult your veterinarian, your pet’s healthcare expert. For technical assistance or to report an adverse drug experience, call 1-800-874-9764. Additional information can be found at www.reconcile.com.
- Caution:
-
Description:
RECONCILE is a chewable, flavored tablet that contains fluoxetine hydrochloride. RECONCILE chewable tablets are available in 8, 16, 32, and 64 mg tablet strengths for oral administration to dogs. The active ingredient in RECONCILE chewable tablets is fluoxetine hydrochloride, a selective serotonin reuptake inhibitor (SSRI). The molecular weight of fluoxetine is 345.79. The structural formula is depicted below.
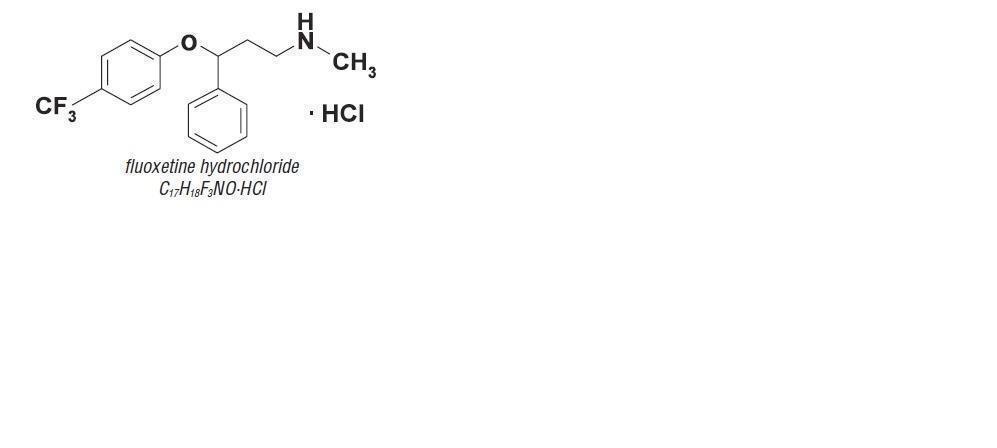
- Indications:
-
Dosage and Administration:
The recommended dose of RECONCILE chewable tablets is 1-2 mg/kg (0.5-0.9 mg/lb) administered once daily, in conjunction with a behavior modification plan. A typical behavior modification plan consists of the pet owner implementing standard training techniques based on principles such as rewarding appropriate behavior; coming and going in a manner that does not elicit inappropriate responses from the dog; and teaching the dog to be content while alone.
Table 1: Recommended Dose of RECONCILE Chewable Tablets
Dog Weight Dog Weight No. of Tablets/Day Tablet Strength (mg) (lb) (kg) 8.8 - 17.6 4.0 - 8.0 1 8 17.7 - 35.2 8.1 - 16.0 1 16 35.3 - 70.4 16.1 - 32.0 1 32 70.5 - 140.8 32.1 - 64.0 1 64 -
SPL UNCLASSIFIED SECTION
The effectiveness and safety of RECONCILE chewable tablets was demonstrated in a field study in client-owned dogs (see EFFECTIVENESS and ADVERSE REACTIONS). At the end of the 8-week study, 73% of dogs treated with RECONCILE chewable tablets showed significant improvement (p=0.010), as compared to behavior modification alone (51%). During the course of therapy, 42% of dogs showed improvement within the first week, which was significantly greater (p=0.005) than with behavior modification alone (18%). The patient’s response to therapy should be monitored. If no improvement is noted within 8 weeks, case management should be reevaluated.
The effectiveness and clinical safety of RECONCILE chewable tablets for long-term use (i.e. for more than 8 weeks) has not been evaluated. RECONCILE chewable tablets were evaluated at the recommended label dose for one year in a laboratory safety study in dogs (see ANIMAL SAFETY).
Professional judgment should be used in monitoring the patient’s response to therapy to determine the need to continue treatment with RECONCILE chewable tablets beyond 8 weeks. To discontinue therapy, it is not necessary to taper or reduce doses because of the long half-life of this product. Continued behavioral modification is recommended to prevent recurrence of the clinical signs.
RECONCILE chewable tablets are readily consumed by dogs or can be administered like other tablet medications, and can be given with or without food.
Professional discretion should be used in determining the need for dose reduction in the event of a possible adverse reaction. Approximately half of patients tolerate a return to the previous dose after 1-2 weeks on a reduced schedule (see ADVERSE REACTIONS).
If a dose is missed, the next scheduled dose should be adminis-tered as prescribed. Do not increase or double the dose.
-
Contraindications:
RECONCILE chewable tablets are contraindicated for use in dogs with epilepsy or a history of seizures. RECONCILE chewable tablets should not be given concomitantly with drugs that lower the seizure threshold (e.g., phenothiazines such as acepromazine or chlorpromazine).
RECONCILE chewable tablets should not be given in combination with a monoamine oxidase inhibitor (MAOI) [e.g., selegiline hydrochloride (L-deprenyl) or amitraz], or within a minimum of 14 days of discontinuing therapy with an MAOI.
RECONCILE chewable tablets are contraindicated in dogs with a known hypersensitivity to fluoxetine HCl or other SSRIs.
Because fluoxetine and its major metabolite, norfluoxetine, have long half-lives, a 6-week washout interval should be observed following discontinuation of therapy with RECONCILE chewable tablets prior to the administration of any drug that may adversely interact with fluoxetine or norfluoxetine.
-
Warnings:
Not for use in humans. Keep out of reach of children. In case of accidental ingestion seek medical attention immediately. In humans, the most common symptoms associated with over dosage include seizures, somnolence, nausea, tachycardia, and vomiting. In case of ingestion by a human, contact a physi-cian immediately. For a copy of the Material Safety Data Sheet (MSDS) or to report adverse reactions call 1-800-874-9764.
-
Precautions:
RECONCILE chewable tablets are not recommended for the treatment of aggression. RECONCILE chewable tablets have not been clinically tested for the treatment of other behav-ioral dis orders. Studies to determine the effects of RECONCILE chewable tablets in breeding, pregnant, or lactating dogs and in patients less than 6 months of age have not been conducted.
Seizures may occur in dogs treated with RECONCILE chewable tablets, even in dogs without a history of epilepsy or seizures (see ADVERSE REACTIONS).
Before prescribing RECONCILE chewable tablets, a comprehensive physical examination should be conducted to rule out causes of inappropriate behavior unrelated to separation anxiety. The examination should include a thorough history and assessment of the patient’s household environment and standard practice laboratory tests as appropriate for the patient’s age and health status. Veterinarians should be familiar with the risks and benefits of the treatment of behavioral disorders in dogs before initiating therapy. Inappropriate use of RECONCILE chewable tablets, i.e. in the absence of a diagnosis or without concurrent behavior modification, may expose the animal to un-necessary adverse reactions and may not provide any lasting benefit of therapy.
RECONCILE chewable tablets have not been evaluated with drugs that affect the cytochrome P450 enzyme system. RECONCILE chewable tablets should be used with caution when co-administered with any drug that affects the cytochrome P450 enzyme system (for example, ketaconazole). Studies to assess the interaction of RECONCILE chewable tablets with tricyclic antidepressants (TCAs) (for example, amitriptyline and clomipramine) have not been conducted. The minimum washout period to transition dogs from TCAs to RECONCILE chewable tablets has not been evaluated. Published pharmaco-kinetic data demonstrates that TCAs are cleared 4 days following discontinuation.1, 2
-
Adverse Reactions:
In two North American multi-site field studies, which included a total of 427 dogs, the following adverse reactions were observed:
Seizures:
In one study, one of 112 dogs in the control group and three of 117 dogs that received RECONCILE chewable tablets experienced the serious adverse reaction of seizures. One of the three dogs treated with RECONCILE chewable tablets experienced two seizures 10 days after the end of therapy. Despite escalating phenobarbital doses, the seizures continued and this dog died in status epilepticus approximately six months after the first seizure. Another of the three dogs treated with RECONCILE chewable tablets had experienced one seizure approximately 1½ years prior to study enrollment immediately after receiving head trauma. No additional seizures were reported to have occurred until 45 days after concluding treatment with RECONCILE chewable tablets. During the 1½-year period since the second seizure, this dogs seizure activity increased from single seizures to cluster seizures despite increasing doses of phenobarbital and the addition of oral potassium bromide and rectal diazepam. The third dog treated with RECONCILE chewable tablets and the control dog experienced one seizure 24 days and 35 days, respectively, after the start of therapy; no anticonvulsant therapy was initiated and no further seizures were reported in either dog.
In the second study, one of 99 dogs treated with RECONCILE chewable tablets and one of 99 dogs treated with the control tablet experienced the serious adverse reaction of seizures 9 and 27 days, respectively, after initiation of therapy. The dog treated with RECONCILE chewable tablets was subsequently diagnosed with vestibular disease and the control dog had a history of recurrent hind leg weakness.
In a European multi-site study, 234 dogs were treated with daily doses of fluoxetine chewable tablets ranging from 0.25 mg/kg to 4 mg/kg. One dog treated with a daily dose of 0.4 mg/kg for one month experienced one seizure one week after discontinuing therapy. No anticonvulsant therapy was initiated and no further seizures were reported.
Weight loss:
Of the dogs in the two North American field studies with body weight measurements throughout the study (n=196 and n=185 in the RECONCILE chewable tablets and control group, respectively), a 5% or greater weight loss (when compared to initial, pre-study body weight) was observed in 58 (29.6%) of dogs treated with RECONCILE chewable tablets and 24 (13.0%) of dogs in the control group. No dogs were withdrawn from clinical studies due to weight loss alone. The following table shows the number of dogs with weight loss, stratified by percent weight loss relative to initial body weight.
Table 2: Dogs with Weight Loss (stratified by percent loss relative to initial body weight)
Treatment Group 5% to <10% Number (%) 10% to <15% Number (%) 15% Number (%) RECONCILE chewable tablets 44 (22.5%) 13 (6.6%) 1a (0.5%) Control 20 (10.8%) 4 (2.2%) 0 (0%) aThis dog lost 20% of its initial body weight and was the same dog that died in status epilepticus.
Other adverse reactions:
Additional adverse reactions observed in dogs treated with RECONCILE chewable tablets at a rate of 1% or greater were:
Table 3: Adverse Reactions Reported in the North American Field Studies
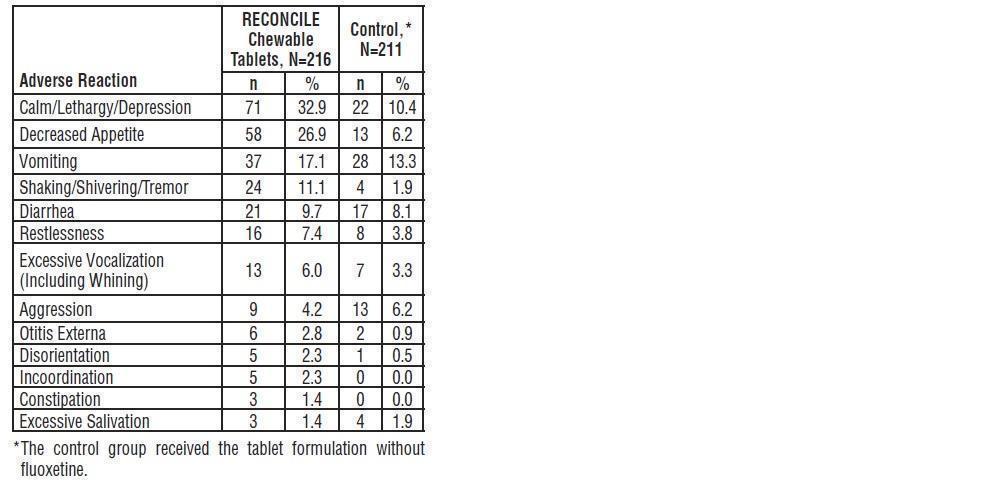
Dose Reduction:
Twenty dogs in the RECONCILE chewable tablet group and five dogs in the control group required a reduction in dose due to unacceptable adverse reactions, generally anorexia, vomiting, shaking and depression. Lowering the dose eliminated or reduced the severity of these adverse reactions in the RECONCILE chewable tablet group only. Resumption of the full dose of RECONCILE chewable tablets resulted in a return of the initial adverse reactions in approximately half of the affected dogs. The majority of these adverse reactions were intermittent and mild. However, one dog experienced recurrence of severe adverse reactions, which necessitated withdrawal from the study for that
dog. Additionally, two dogs required a second dose reduction of RECONCILE chewable tablets. Effectiveness was maintained in a majority of those dogs in which a dose reduction was necessary.Post Approval Experience (Rev. 2010):
The following adverse events are based on post-approval adverse drug experience reporting with RECONCILE chewable tablets. Not all adverse reactions are reported to FDA CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using this data.
The following adverse events are listed in decreasing order of reported frequency: decreased appetite, depression/lethargy, shaking/shivering/tremor, vomiting, restlessness and anxiety, seizures, aggression, diarrhea, mydriasis, vocalization, weight loss, panting, confusion, incoordination, and hypersalivation.
For a copy of the Safety Data Sheet (SDS) or to report suspected adverse drug events, contact Pegasus Laboratories at 1-800- 874-9764. For additional information about adverse drug expe-rience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/AnimalVeterinary/SafetyHealth.
-
Clinical Pharmacology:
Fluoxetine exerts its effect by inhibiting the reuptake of serotonin at the pre-synaptic neuron. Fluoxetine does not act as a sedative. Fluoxetine is well absorbed after oral administration (~72%). It is largely metabolized in the liver by cytochrome P-450 enzyme system to norfluoxetine, an equipotent SSRI that contributes to the efficacy of RECONCILE chewable tablets.
After a single dose, and also at steady state, calculations were made as follows:
Table 4: Single Dose* Pharmacokinetic Parameters of Fluoxetine Hydrochloride (mean ± standard error).
AUC0-∞ (µghr/mL) cmax (ng/mL) Tmax
(hr)
T1/2
(hr)
T1/2 Range (hr) Fluoxetine 1.388 (±0.137) 126.6 (±12.3) 1.8 (±0.2) 6.2 (±0.8) 3.0 - 12.9 Norfluoxetine 11.44 (±0.74) 138.3 (±9.6) 12.8 (±1.7) 49 (±3) 33.0 - 64.0 *approximately 2 mg/kg body weight
In a 21-day study, fluoxetine was administered daily at a dose of 0.75, 1.5 and 3.0 mg/kg to laboratory Beagles. The maximum plasma concentration (Cmax) and area under the plasma concentration time curve (AUC) for fluoxetine were approximately dose proportional between 0.75 and 1.5 mg/kg, with a greater than dose proportional increase at 3 mg/kg. Norfluoxetine Cmax and AUC were generally dose proportional.
Although steady state appeared to be reached within 10 days in the 21-day study, a continuous increase in trough concentrations was observed in a one year, multiple-dose laboratory safety study. In this study, dogs administered a 1 mg/kg dose of fluoxetine had plasma fluoxetine concentrations that continued to increase over the one-year dosing period. A similar increase in concentrations was observed with norfluoxetine. This phenomenon was not observed at higher doses. During the one-year dosing interval and the subsequent two-month recovery period, there were no changes in the nature and frequency of adverse reactions observed as compared to those seen by Day 28 of fluoxetine administration.
-
Effectiveness:
In one randomized multi-centered, double-blinded, vehicle- controlled study of 8 weeks duration, 229 dogs were evaluated at 34 investigative sites in the United States and Canada. One hundred seventeen dogs were randomized to 1-2 mg/kg/day of RECONCILE chewable tablets and 112 dogs were random-ized to the control group. Both groups underwent concurrent behavior modification. In seven of the eight weeks, the percentage of dogs with improved overall separation anxiety scores was significantly higher (p ≤ 0.05) among dogs treated with RECONCILE chewable tablets compared to dogs that received the control tablet. At the end of the study, 73% of dogs treated with RECONCILE chewable tablets showed significant improve-ment (p=0.010) as compared to 51% of dogs treated with behavior modification alone.
Dogs treated with RECONCILE chewable tablets also showed improvement in destructive behavior, excessive vocalization, and restlessness over dogs that received the control tablet. In addition, dogs in both groups experienced improvement in inappropriate urination, inappropriate defecation, excessive salivation, excessive licking/grooming, shaking/shivering and depression. Overall separation anxiety severity scores improved more rapidly for dogs taking RECONCILE chewable tablets than those dogs receiving the control tablet. The same effect was also noted for the individual scores for excessive vocalization and depression.
Animal Safety:
In a one-year laboratory safety study, dogs were dosed daily at 1, 4.5, and 20 mg/kg/day of a gelatin capsule filled with fluoxetine powder. Based upon the results of a relative bioavailability study comparing the fluoxetine-filled capsule versus the REC-ONCILE chewable tablets, the corresponding equivalent doses were 0.87, 3.9 and 17.4 mg/kg/day of RECONCILE chewable tablets (where the average ratio of fluoxetine AUC values for RECONCILE chewable tablets/fluoxetine-filled capsule = 1.15).
Three of five female dogs in the 20 mg/kg group, died or were euthanatized during the first six months of the study. The high dose was decreased to 10 mg/kg/day (equivalent to 8.7 mg/kg/day of RECONCILE chewable tablets) for the last six months of the treatment, and all remaining dogs completed the study. One dog in the 1 mg/kg group (equivalent to 0.87 mg/kg/day of RECONCILE chewable tablets) and two dogs in the 20 mg/kg group (equivalent to 17.4 mg/kg/day of RECONCILE chewable tablets) experienced a seizure. Aggressive behavior, ataxia, salivation at dosing, hyperesthesia, nystagmus, thin body condition, weakness, lethargy, diarrhea and head tilt were also noted in the high dose group. Anorexia, tremors, decreased pupillary light response, mydriasis, vomiting, and decreased weight gain were observed in all treatment groups, but occurred more frequently in the high dose group. With the exception of decreased weight gain, all abnormal observations resolved by the end of a two-month recovery period. Evidence of phospholipidosis was noted in the lung, liver, adrenal glands, lymph nodes, spleen, retina and white blood cells of all groups, which resolved during the recovery period. Fluoxetine caused no marked or consistent effects on hematology, blood chemistries or urinalysis. Brady-cardia was absent on the electrocardiogram in the control and lowest dose groups, but was mildly present in a dose-dependent manner in the two higher dose groups. There were no effects noted on gross organ examination.
- Storage Information:
- How Supplied:
- SPL UNCLASSIFIED SECTION
-
References:
1 Plumb DC. Amitriptyline. Veterinary Drug Handbook 5th Edition (Pocket Edition). Iowa State Press. Ames, IA. Page 39, 2002.
2 Hewson CJ, et.al. The pharmacokinetics of clomipramine and desmethylclomipramine in dogs: parameter estimates following a single oral dose and 28 consecutive daily doses of clomipramine. J Vet Pharmacol Therap 21:214-222, 1998. - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RECONCILE
fluoxetine hydrochloride tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 49427-342 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUOXETINE HYDROCHLORIDE (UNII: I9W7N6B1KJ) (FLUOXETINE - UNII:01K63SUP8D) FLUOXETINE 32 mg Product Characteristics Color brown (speckled tan to brown) Score no score Shape ROUND Size 13mm Flavor MEAT (Beef Flavor) Imprint Code 4207 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49427-342-57 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141272 04/01/2018 Labeler - Pegasus Laboratories, Inc. (108454760) Registrant - Pegasus Laboratories, Inc. (108454760) Establishment Name Address ID/FEI Business Operations Pegasus Laboratories, Inc. 108454760 analysis, manufacture, label
Trademark Results [RECONCILE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 RECONCILE 98060787 not registered Live/Pending |
Helena Holding Company 2023-06-27 |
 RECONCILE 78366279 3330512 Live/Registered |
PEGASUS LABORATORIES, INC. 2004-02-11 |
 RECONCILE 77437428 3553274 Dead/Cancelled |
Loraas, Jason A 2008-04-01 |
 RECONCILE 77188685 3469031 Dead/Cancelled |
Reconcile New Orleans, Inc. 2007-05-23 |
 RECONCILE 77188642 3469030 Dead/Cancelled |
Reconcile New Orleans, Inc. 2007-05-23 |
 RECONCILE 76186173 2591002 Dead/Cancelled |
MICRO FOCUS IP DEVELOPMENT LIMITED 2000-12-26 |
 RECONCILE 74564523 not registered Dead/Abandoned |
MITSUBISHI ELECTRIC RESEARCH LABORATORIES, INC. 1994-08-23 |
 RECONCILE 74393278 not registered Dead/Abandoned |
MITSUBISHI ELECTRIC RESEARCH LABORATORIES, INC. 1993-05-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
