ULTRAVIST- iopromide injection
Ultravist by
Drug Labeling and Warnings
Ultravist by is a Prescription medication manufactured, distributed, or labeled by Bayer HealthCare Pharmaceuticals Inc., Bayer Schering Pharma AG, Bayer AG. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ULTRAVIST PHARMACY BULK PACKAGE safely and effectively. See full prescribing information for ULTRAVIST PHARMACY BULK PACKAGE.
ULTRAVIST (iopromide) Injection, for intravenous or intra-arterial use
Initial U.S. Approval: 1995WARNING: NOT FOR INTRATHECAL USE
See full prescribing information for complete boxed warning.
Inadvertent intrathecal administration may cause death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia, and brain edema.
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
ULTRAVIST (iopromide) Injection is a radiographic contrast agent indicated for:
- Cerebral arteriography and peripheral arteriography (300 mg Iodine per mL) (1.1)
- Coronary arteriography and left ventriculography, visceral angiography and aortography (370 mg Iodine per mL) (1.1)
- Excretory urography (300 mg Iodine per mL) (1.2)
- Contrast computed tomography (CT) imaging of head and body (300 mg Iodine per mL and 370 mg Iodine per mL) (1.2)
DOSAGE AND ADMINISTRATION
ULTRAVIST PHARMACY BULK PACKAGE IS NOT FOR DIRECT INFUSION
Carefully individualize the volume and concentration of ULTRAVIST Injection to be used for a vascular procedure, according to the specific dosing tables. Adjust the dose accounting for factors such as age, body weight, size of the vessel and the rate of blood flow within the vessel. (2)
DOSAGE FORMS AND STRENGTHS
ULTRAVIST Injection PHARMACY BULK PACKAGE is available in two strengths: 300 mg Iodine per mL; 370 mg Iodine per mL. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Anaphylactoid Reactions: Life-threatening or fatal anaphylactoid reactions may occur during or after ULTRAVIST administration, particularly in patients with allergic disorders. (5.1)
- Acute Renal Failure: Acute renal failure may occur following ULTRAVIST administration, particularly in patients with renal insufficiency, diabetes, multiple myeloma. Exercise caution and use the lowest necessary dose of ULTRAVIST in patients with renal dysfunction. (5.2)
- Cardiovascular Reactions: Hemodynamic disturbances including shock and cardiac arrest may occur during or shortly after administration of ULTRAVIST. (5.3)
- Thromboembolic Complications: Angiography may be associated with local and distal organ damage, ischemia, thromboembolism and organ failure. In angiographic procedures, consider the possibility of dislodging plaques or damaging or perforating the vessel wall. The physicochemical properties of the contrast agent, the dose and the speed of injection can influence the reactions. (5.4)
ADVERSE REACTIONS
Most common adverse reactions (>1%) are headache, nausea, injection site and infusion site reactions, vasodilatation, vomiting, back pain, urinary urgency, chest pain, pain, dysgeusia and abnormal vision. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Bayer HealthCare Pharmaceuticals Inc. at 1-888-842-2937 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
- Lactation: Advise lactating women that interruption of breast feeding is not necessary, however, a lactating woman may consider interrupting breastfeeding and pumping and discarding breast milk for 12 to 24 hours after ULTRAVIST Injection administration to minimize exposure to the breastfed infant (8.2)
- The safety and efficacy of ULTRAVIST Injection have been established in the pediatric population over 2 years of age. (8.4)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: NOT FOR INTRATHECAL USE
1 INDICATIONS AND USAGE
1.1 Intra-Arterial Procedures*
1.2 Intravenous Procedures*
2 DOSAGE AND ADMINISTRATION
2.1 Intra-Arterial Procedures
2.2 Intravenous Procedures
2.3 Pediatric Dosing
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Anaphylactoid Reactions
5.2 Contrast Induced Acute Kidney Injury
5.3 Cardiovascular Reactions
5.4 Thromboembolic Complications
5.5 Reactions in Patients with Hyperthyroidism, Pheochromocytoma, or Sickle Cell Disease
5.6 Extravasation
5.7 Increased Radiation Exposure
5.8 Interference with Image Interpretation
5.9 Severe Cutaneous Adverse Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
6.3 Pediatrics
7 DRUG INTERACTIONS
7.1 Drug-Drug Interactions
7.2 Drug-Laboratory Test Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: NOT FOR INTRATHECAL USE
Inadvertent intrathecal administration may cause death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia, and brain edema. [See Contraindications (4).]
-
1 INDICATIONS AND USAGE
ULTRAVIST® Injection is an iodinated contrast agent indicated for:
1.1 Intra-Arterial Procedures*
- 300 mg Iodine per mL for cerebral arteriography and peripheral arteriography
- 370 mg Iodine per mL for coronary arteriography and left ventriculography, visceral angiography, and aortography
1.2 Intravenous Procedures*
- 300 mg Iodine per mL for excretory urography
- 300 mg Iodine per mL and 370 mg Iodine per mL for contrast Computed Tomography (CT) of the head and body (intrathoracic, intra-abdominal and retroperitoneal regions) for the evaluation of neoplastic and non-neoplastic lesions. The usefulness of contrast enhancement for the investigation of the retrobulbar space and of low grade or infiltrative glioma has not been demonstrated.
*For information on the concentrations and doses for the Pediatric Population [see Dosage and Administration (2.3) and Use in Specific Populations (8.4)].
-
2 DOSAGE AND ADMINISTRATION
- ULTRAVIST PHARMACY BULK PACKAGE is not for direct infusion.
- Visually inspect ULTRAVIST for particulate matter and/or discoloration, whenever solution and container permit. Do not administer ULTRAVIST if particulate matter (including crystals) and/or discoloration is observed or containers are defective. As ULTRAVIST Injection is a highly concentrated solution, crystallization (milky-cloudy appearance and/or sediment at bottom, or floating crystals) may occur.
- Use sterile technique for all handling and administration of ULTRAVIST.
- Determine the volume and concentration of ULTRAVIST Injection to be used taking into account factors such as age, body weight, size of the vessel and the rate of blood flow within the vessel; consider also extent of opacification required, structure(s) or area to be examined, disease processes affecting the patient, and equipment and technique to be employed. Specific dose adjustments for age, gender, weight and renal function have not been studied for ULTRAVIST Injection. As with all iodinated contrast agents, lower doses may have less risk. The efficacy of ULTRAVIST Injection below doses recommended has not been established.
- Administer ULTRAVIST at or close to body temperature.
- Do not mix or inject ULTRAVIST Injection in intravenous administration lines containing other drugs or total nutritional admixtures. ULTRAVIST can be mixed with saline when used in a power injector suitable for simultaneous injection of contrast and saline [see Dosage and Administration (2.2)].
- The maximum recommended total dose of iodine in adults is 86 grams; a maximum recommended total dose of iodine has not been established for pediatric patients.
- Hydrate patients, as appropriate, prior to and following the administration of ULTRAVIST [see Warnings and Precautions (5.2)].
2.1 Intra-Arterial Procedures
The volume and rate of injection of the contrast agent will vary depending on the injection site and the area being examined. Inject contrast at rates approximately equal to the flow rate in the vessel being injected.
- Cerebral Arteriography (300 mg Iodine per mL), Coronary Arteriography and Left Ventriculography (370 mg Iodine per mL), Peripheral Arteriography (300 mg Iodine per mL): see Table 1.
- Aortography and Visceral Angiography (370 mg Iodine per mL):
- Use a volume and rate of contrast injection proportional to the blood flow and related to the vascular and pathological characteristics of the specific vessels being studied. Do not exceed 225 mL as total dose for the procedure.
Table 1: Suggested Single Injection Doses for Adult Intra-Arterial Procedures Cerebral Arteriography
(300 mg Iodine per mL)
Peripheral Arteriography
(300 mg Iodine per mL)
Coronary Arteriography and Left Ventriculography
(370 mg Iodine per mL)
- Intra-Arterial Injection Sites
Carotid Arteries
Vertebral Arteries
Aortic Arch Injection (4 vessel study)
3 ml to 12 mL
4 ml to 12 mL
20 ml to 50 mL
-
-
-
-
-
-
Right Coronary Artery
Left Coronary Artery
Left Ventricle
-
-
-
-
-
-
3 ml to 14 mL
3 ml to 14 mL
30 ml to 60 mL
Subclavian or Femoral Artery
Aortic Bifurcation (distal runoff)
-
-
5 ml to 40 mL
25 ml to 50 mL
-
-
Maximum Total Dose
150 mL
250 mL
225 mL
2.2 Intravenous Procedures
- Contrast Computed Tomography (CT) (300 mg Iodine per mL and 370 mg Iodine per mL) and Excretory Urography (300 mg Iodine per mL): see Table 2.
Table 2: Suggested ULTRAVIST Injection Dosing for Adult Intravenous Contrast Administration Excretory Urography (300 mg Iodine per mL)
Contrast Computed Tomography (300 mg Iodine per mL)
Contrast Computed Tomography
(370 mg Iodine per mL)Excretory Urography
Approximately 300 mg Iodine per kg body wt. (Adults with normal renal function)
-
-
Head
-
50 ml to 200 mL
41 ml to 162 mL
Body
Single Contrast Phase
Bolus Injection
-
50 ml to 200 mL
41 ml to 162 mL
Rapid Infusion
-
100 ml to 200 mL
81 ml to 162 mL
Body
Multiple Phase Contrast
50 ml to 200 mL total volume
Phase 1: 100% contrast
Phase 2: 20-60% contrast
using a power injector suitable for simultaneous injection41 ml to 162 mL total volume
Phase 1: 100% contrast
Phase 2: 20-60% contrast
using a power injector suitable for simultaneous injectionMaximum Total Dose
100 mL (30 g iodine)
200 mL (60 g iodine)
162 mL (60 g iodine)
2.3 Pediatric Dosing
The recommended dose in children over 2 years of age for the following evaluations is:
- Intra-arterial:
- Cardiac chambers and related arteries (370 mg Iodine per mL):
- Inject 1 to 2 milliliters per kilogram (mL/kg). Do not exceed 4 mL/kg as total dose.
- Intravenous:
- Contrast Computerized Tomography or Excretory Urography (300 mg Iodine per mL):
- Inject 1 to 2 mL/kg. Do not exceed 3 mL/kg as total dose.
The safety and efficacy relationships of other doses, concentrations or procedures have not been established [see Use in Specific Populations (8.4) and Clinical Pharmacology (12.3)].
-
3 DOSAGE FORMS AND STRENGTHS
ULTRAVIST Injection is a nonionic, sterile, clear, colorless to slightly yellow, odorless, pyrogen-free aqueous solution of iopromide, containing 2.42 mg/mL tromethamine buffer and 0.1 mg/mL edetate calcium disodium stabilizer.
ULTRAVIST Injection PHARMACY BULK PACKAGE is available in two strengths:
300 mg Iodine per mL provides 623.4 mg/mL iopromide
370 mg Iodine per mL provides 768.86 mg/mL iopromide
-
4 CONTRAINDICATIONS
- Do not administer ULTRAVIST Injection intrathecally. Inadvertent intrathecal administration may cause death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia, and brain edema.
- Preparatory dehydration (for example, prolonged fasting and the administration of a laxative) before ULTRAVIST Injection is contraindicated in pediatric patients because of risk of acute renal failure.
-
5 WARNINGS AND PRECAUTIONS
5.1 Anaphylactoid Reactions
Life-threatening or fatal, anaphylactoid reactions, may occur during or after ULTRAVIST administration. Manifestations include respiratory arrest, laryngospasm, bronchospasm, angioedema, and shock. Increased risk is associated with a history of previous reaction to a contrast agent (3-fold), a known sensitivity to iodine and known allergic disorders (that is, bronchial asthma, hay fever and food allergies) or other hypersensitivities (2-fold). Exercise extreme caution when considering the use of iodinated contrast agents in patients with these histories or disorders.
Emergency facilities and personnel trained in the treatment of anaphylactoid reactions should be available for at least 30 to 60 minutes after ULTRAVIST administration.
5.2 Contrast Induced Acute Kidney Injury
Acute kidney injury, including renal failure, may occur after intravascular administration of ULTRAVIST. Risk factors include: pre-existing renal insufficiency, dehydration, diabetes mellitus, congestive heart failure, advanced vascular disease, elderly age, concomitant use of nephrotoxic or diuretic medications, multiple myeloma / paraproteinemia, repetitive and/or large doses of ULTRAVIST.
Use the lowest necessary dose of ULTRAVIST in patients with renal impairment. Hydrate patients, as appropriate, prior to and following ULTRAVIST administration.
5.3 Cardiovascular Reactions
ULTRAVIST increases the circulatory osmotic load and may induce acute or delayed hemodynamic disturbances in patients with congestive heart failure, severely impaired renal function, combined renal and hepatic disease, combined renal and cardiac disease, particularly when repetitive and/or large doses are administered [see Drug Interactions (7)].
Among patients who have had cardiovascular reactions, most deaths occurred from the start of injection to 10 minutes later; the main feature was cardiac arrest with cardiovascular disease as the main underlying factor. Isolated reports of hypotensive collapse and shock have been published.
The administration of ULTRAVIST may cause pulmonary edema in patients with heart failure. Based upon published reports, deaths from the administration of iodinated contrast agents range from 6.6 per 1 million (0.00066 percent) to 1 in 10,000 patients (0.01 percent). Observe patients with preexisting cardiovascular disease for several hours following ULTRAVIST administration.
5.4 Thromboembolic Complications
- Angiography may be associated with local and distal organ damage, ischemia, thromboembolism and organ failure including stroke, brachial plexus palsy, chest pain, myocardial infarction, sinus arrest, hepato-renal function abnormalities. For these reasons, meticulous angiographic techniques are recommended, including close attention to guide wire and catheter manipulation, use of manifold systems and/or three-way stopcocks, frequent catheter flushing with heparinized saline solutions and minimizing the length of the procedure. In angiographic procedures, consider the possibility of dislodging plaques or damaging or perforating the vessel wall with resultant pseudoaneurysms, hemorrhage at puncture site, dissection of coronary artery during catheter manipulations and contrast agent injection. The physicochemical properties of the contrast agent, the dose and the speed of injection can influence the reactions. Test injections to ensure proper catheter placement are suggested. Increased thrombosis and activation of the complement system has also occurred. Specialized personnel, and adequate equipment and facilities for immediate resuscitation and cardioversion are necessary. Monitor electrocardiograms and vital signs throughout the procedure.
- Exercise care when performing venography in patients with suspected thrombosis, phlebitis, severe ischemic disease, local infection, venous thrombosis or a totally obstructed venous system.
- Clotting may occur when blood remains in contact with syringes containing iodinated contrast agents.
- Avoid angiography whenever possible in patients with homocysteinuria because of the risk of inducing thrombosis and embolism [see Clinical Pharmacology (12.2)].
5.5 Reactions in Patients with Hyperthyroidism, Pheochromocytoma, or Sickle Cell Disease
Thyroid storm in patients with hyperthyroidism. Thyroid storm has occurred after the intravascular use of iodinated contrast agents in patients with hyperthyroidism, or with an autonomously functioning thyroid nodule. Evaluate the risk in such patients before use of any iodinated contrast agent.
Hypertensive crises in patients with pheochromocytoma. Administer iodinated contrast agents with extreme caution in patients with known or suspected pheochromocytoma. Inject the minimum amount of contrast necessary. Assess the blood pressure throughout the procedure, and have measures for treatment of a hypertensive crisis readily available.
Sickle cell disease. Contrast agents may promote sickling in individuals who are homozygous for sickle cell disease when administered intravascularly.
5.6 Extravasation
Extravasation of ULTRAVIST Injection may cause tissue necrosis and/or compartment syndrome, particularly in patients with severe arterial or venous disease.
5.7 Increased Radiation Exposure
The decision to use contrast enhancement is associated with risk and increased radiation exposure. Use contrast after a careful evaluation of clinical, other radiologic data, and the results of non-contrast CT findings, taking into account the increased radiation dose and other risks.
5.8 Interference with Image Interpretation
As with other iodinated contrast agents, the use of ULTRAVIST Injection may obscure some lesions which were seen on non-contrast CT scans. Calcified lesions are less likely to enhance. The enhancement of tumors after therapy may decrease. The opacification of the inferior vermis following contrast agent administration has resulted in false-positive diagnosis. Cerebral infarctions of recent onset may be better visualized with contrast enhancement. However, older infarctions may be obscured by the contrast agent.
In patients with normal blood-brain barriers and renal failure, iodinated contrast agents have been associated with blood-brain barrier disruption and accumulation of contrast in the brain. Accumulation of contrast in the brain also occurs in patients where the blood-brain barrier is known or suspected to be disrupted.
5.9 Severe Cutaneous Adverse Reactions
Severe cutaneous adverse reactions (SCAR) may develop from 1 hour to several weeks after intravascular contrast agent administration. These reactions include Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), acute generalized exanthematous pustulosis (AGEP) and drug reaction with eosinophilia and systemic symptoms (DRESS). Reaction severity may increase and time to onset may decrease with repeat administration of contrast agent; prophylactic medications may not prevent or mitigate severe cutaneous adverse reactions. Avoid administering ULTRAVIST to patients with a history of a severe cutaneous adverse reaction to ULTRAVIST.
-
6 ADVERSE REACTIONS
The most important adverse drug reactions in patients receiving ULTRAVIST are anaphylactoid shock, contrast induced acute kidney injury, coma, cerebral infarction, stroke, brain edema, convulsion, arrhythmia, cardiac arrest, myocardial ischemia, myocardial infarction, cardiac failure, bradycardia, cyanosis, hypotension, shock, dyspnea, pulmonary edema, respiratory insufficiency and aspiration.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect or predict the rates observed in practice.
The following table of incidence of reactions is based upon controlled clinical trials in which ULTRAVIST Injection was administered to 1142 patients. This listing includes all reported adverse reactions regardless of attribution.
Adverse reactions are listed by System Organ Class and in decreasing order of occurrence for rates greater than 1% in the ULTRAVIST group: see Table 3.
Table 3: ADVERSE REACTIONS REPORTED IN > 1% OF PATIENTS WHO RECEIVED ULTRAVIST INJECTION IN CLINICAL TRIALS System Organ Class
Adverse Reaction
ULTRAVIST Injection
N=1142 (%)
Nervous system disorders
Headache
46 (4)
Dysgeusia
15 (1.3)
Eye disorders
Abnormal Vision
12 (1.1)
Cardiac disorders
Chest pain
18 (1.6)
Vascular disorders
Vasodilatation
30 (2.6)
Gastrointestinal disorders
Nausea
42 (3.7)
Vomiting
22 (1.9)
Musculoskeletal and connective tissue disorders
Back pain
22 (1.9)
Renal and urinary disorders
Urinary urgency
21 (1.8)
General disorders and administration site conditions
Injection site and infusion site reactions (hemorrhage, hematoma, pain, edema, erythema, rash)
41 (3.7)
Pain
13 (1.4)
One or more adverse reactions were recorded in 273 of 1142 (24%) patients during the clinical trials, coincident with the administration of ULTRAVIST Injection or within the defined duration of the study follow-up period (24–72 hours). ULTRAVIST Injection is often associated with sensations of warmth and/or pain.
Serious, life-threatening and fatal reactions have been associated with the administration of iodine-containing contrast media, including ULTRAVIST Injection. In clinical trials 7/1142 patients given ULTRAVIST Injection died 5 days or later after drug administration. Also, 10/1142 patients given ULTRAVIST Injection had serious adverse events.
The following adverse reactions were observed in ≤1% of the subjects receiving ULTRAVIST Injection:
Cardiac disorders: atrioventricular block (complete), bradycardia, ventricular extrasystole
Gastrointestinal disorders: abdominal discomfort, abdominal pain, abdominal pain upper, constipation, diarrhea, dry mouth, dyspepsia, gastrointestinal disorder, gastrointestinal pain, salivation increased, stomach discomfort, rectal tenesmus
General disorders and administration site conditions: asthenia, chest discomfort, chills, excessive thirst, extravasation, feeling hot, hyperhidrosis, malaise, edema peripheral, pyrexia
Immune system disorders: asthma, face edema
Investigations: blood lactate dehydrogenase increased, blood urea increased, hemoglobin increased, white blood cell count increased
Musculoskeletal and connective tissue disorders: arthralgia, musculoskeletal pain, myasthenia, neck pain, pain in extremity
Nervous system disorders: agitation, confusion, convulsion, dizziness, hypertonia, hypesthesia, incoordination, neuropathy, somnolence, speech disorder, tremor, paresthesia, visual field defect
Psychiatric disorders: anxiety
Renal and urinary disorders: dysuria, renal pain, urinary retention
Respiratory, thoracic and mediastinal disorders: apnea, cough increased, dyspnea, hypoxia, pharyngeal edema, pharyngitis, pleural effusion, pulmonary hypertension, respiratory disorder, sore throat
Skin and subcutaneous tissue disorders: erythema, pruritus, rash, urticaria
Vascular disorders: coronary artery thrombosis, flushing, hypertension, hypotension, peripheral vascular disorder, syncope, vascular anomaly
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of ULTRAVIST Injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Adverse reactions reported in foreign postmarketing surveillance and other trials with the use of ULTRAVIST Injection include:
Cardiac disorders: cardiac arrest, ventricular fibrillation, atrial fibrillation, tachycardia, palpitations, congestive heart failure, myocardial infarction, angina pectoris
Ear and labyrinth disorders: vertigo, tinnitus
Endocrine disorders: hyperthyroidism, thyrotoxic crisis, hypothyroidism; Thyroid function tests indicative of hypothyroidism or transient thyroid suppression have been uncommonly reported following iodinated contrast administration to adult and pediatric patients, including infants. Some patients were treated for hypothyroidism.
Eye disorders: mydriasis, lacrimation disorder
Gastrointestinal disorders: dysphagia, swelling of salivary glands
Immune system disorders: anaphylactoid reaction (including fatal cases), respiratory arrest, anaphylactoid shock, angioedema, laryngeal edema, laryngospasm, bronchospasm, hypersensitivity
Musculoskeletal and connective tissue disorders: compartment syndrome in case of extravasation
Nervous system disorders: cerebral ischemia/infarction, paralysis, paresis, transient cortical blindness, aphasia, coma, unconsciousness, amnesia, hypotonia, aggravation of myasthenia gravis symptoms
Renal and urinary disorders: renal failure, hematuria
Respiratory, thoracic and mediastinal disorders: pulmonary edema, acute respiratory distress syndrome, asthma
Skin and subcutaneous tissue disorders: Reactions range from mild (e.g. rash, erythema, pruritus, urticaria and skin discoloration) to severe [e.g. Stevens-Johnson Syndrome and toxic epidermal necrolysis (SJS/TEN), acute generalized exanthematous pustulosis (AGEP) and drug reaction with eosinophilia and systemic symptoms (DRESS)].
Vascular disorders: vasospasm
6.3 Pediatrics
The overall character, quality, and severity of adverse reactions in pediatric patients are generally similar to those reported in adult patients. Additional adverse reactions reported in pediatric patients from foreign marketing surveillance or other information are: epistaxis, angioedema, migraine, joint disorder (effusion), muscle cramps, mucous membrane disorder (mucosal swelling), conjunctivitis, hypoxia, fixed eruptions, vertigo, diabetes insipidus, and brain edema [see Use in Specific Populations (8.4)].
-
7 DRUG INTERACTIONS
7.1 Drug-Drug Interactions
In patients with renal impairment, biguanides can cause lactic acidosis. ULTRAVIST appears to increase the risk of biguanide induced lactic acidosis, possibly as a result of worsening renal function [see Warnings and Precautions (5.2)].
Patients on beta-blockers may be unresponsive to the usual doses of epinephrine used to treat allergic reactions. Because of the risk of hypersensitivity reactions, use caution when administering iodinated contrast agents to patients taking beta-blockers.
Interleukins are associated with an increased prevalence of delayed hypersensitivity reactions after iodinated contrast agent administration. These reactions include fever, chills, nausea, vomiting, pruritus, rash, diarrhea, hypotension, edema, and oliguria.
Renal toxicity has been reported in a few patients with liver dysfunction who were given an oral cholecystographic agent followed by intravascular contrast agents. Administration of any intravascular contrast agent should therefore be postponed in patients who have recently received a cholecystographic contrast agent.
Do not mix other drugs with ULTRAVIST Injection except for saline[see Dosage and Administration (2.2)].
7.2 Drug-Laboratory Test Interactions
Thyroid Function Tests:
The results of protein bound iodine and radioactive iodine uptake studies, which depend on iodine estimation, will not accurately reflect thyroid function for at least 16 days following administration of iodinated contrast agents. However, thyroid function tests which do not depend on iodine estimations, for example, T3 resin uptake and total or free thyroxine (T4) assays are not affected.
Laboratory Assay of Coagulation Parameters, Fibrinolysis and Complement System:
The effect of iopromide on coagulation factors in in vitro assays increased with the administered dose. Coagulation, fibrinolysis and complement activation were evaluated with standard citrated human plasma in the following assays: thrombin time, thrombin coagulase time, calcium thromboplastin time, partial thromboplastin time, plasminogen, thrombin, alpha-2 antiplasmin and factor XIIa activity. Thrombin inhibition was almost complete. Data on reversibility are not available. The thrombin time increased from approximately 20 seconds at an iopromide concentration of 10 mg Iodine per mL, up to 100 seconds at an iopromide concentration of 70 mg Iodine per mL.
The PTT increased from approximately 50 seconds at an iopromide concentration of 10 mg Iodine per mL, up to approximately 100 seconds at an iopromide concentration of 70 mg Iodine per mL. A similar increase was noted in the thrombin coagulase time. Lesser effects were noted in the calcium thromboplastin time. Coagulation time increased from 13.5 to 23 seconds at the highest iopromide concentration of 70 mg Iodine per mL. The Hageman factor split products decreased by about 20% over the range of 10 to 70 mg Iodine per mL of iopromide. Plasminogen was relatively stable. There was no evidence of activation of fibrinolysis. The complement alternate pathway was activated. Factor B conversion increased in a dose dependent manner. The duration of these effects was not studied.
In vitro studies with human blood showed that iopromide had a slight effect on coagulation and fibrinolysis. No Factor XIIa formation could be demonstrated. The complement alternate pathway also can be activated.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data on ULTRAVIST Injection use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Iopromide crosses the placenta and reaches fetal tissues in small amounts (see Data). In animal reproduction studies, intravenous administration of iopromide to pregnant rats and rabbits during organogenesis at doses up to 0.35 and 0.7 times, respectively, the maximum recommended human dose based on body surface area resulted in no relevant adverse developmental effects (see Data). .
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Human Data
Limited case reports demonstrate that intravenously administered iodinated contrast agents, including iopromide, cross the placenta and are visualized in the digestive tract of exposed infants after birth.
Animal Data
Reproduction studies performed with intravenous iopromide in rats (day 6 to 15 of gestation) and rabbits (day 6 to 18 of gestation) at doses up to dose levels of 0, 0.37, 1.11 and 3.7 g I/iodine per kg (2.2 times the maximum recommended dose for a 50 kg human, or approximately 0.7corresponding doses up to 0.35 times the(rats) and 0.7 times (rabbits) the maximum human recommended dose following normalization of the data tobased on body surface area estimates) have revealed no evidence of direct harm to the fetus. Embryolethality. Iopromide was not teratogenic at any dose level in rats and rabbits and embryolethality was observed in rabbits that received 3.7 g I/kgiodine perkg, but this was considered to have been secondary to maternal toxicity.
8.2 Lactation
Risk Summary
There are no data on the presence of iopromide in human milk, the effects on the breastfed infant, or the effects on milk production. Iodinated contrast agents are poorly excreted into human milk and are poorly absorbed by the gastrointestinal tract of a breastfed infant. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ULTRAVIST Injection and any potential adverse effects on the breastfed infant from ULTRAVIST Injection or from the underlying maternal condition (see Clinical Considerations).
Clinical Considerations
Interruption of breastfeeding after exposure to iodinated contrast agents is not necessary because the potential exposure of the breastfed infant to iodine is small. However, a lactating woman may consider interrupting breastfeeding and pumping and discarding breast milk for 12 to 24 hours (approximately 5 elimination half-lives) after ULTRAVIST Injection administration in order to minimize drug exposure to a breast fed infant.
8.4 Pediatric Use
The safety and efficacy of ULTRAVIST Injection have been established in the pediatric population over 2 years of age. Use of ULTRAVIST Injection in these age groups is supported by evidence from adequate and well controlled studies of ULTRAVIST Injection in adults and additional safety data obtained in literature and other reports in a total of 274 pediatric patients. Of these, there were 131 children (2–12 years), 57 adolescents, and 86 children of unreported or other ages. There were 148 females, 94 males and 32 in whom gender was not reported. The racial distribution was: Caucasian 93 (33.9%), Black 1 (0.4%), Asian 6 (2.2%), and unknown 174 (63.5%). These patients were evaluated in intra-arterial coronary angiographic (n=60), intravenous contrast computerized tomography (CT) (n=87), excretory urography (n=99) and 28 other procedures.
In these pediatric patients, a concentration of 300 mg Iodine per mL was employed for intravenous contrast CT or excretory urography. A concentration of 370 mg Iodine per mL was employed for intra-arterial and intracardiac administration in the radiographic evaluation of the heart cavities and major arteries. Most pediatric patients received initial volumes of 1–2 mL/kg.
Optimal doses of ULTRAVIST Injection have not been established because different injection volumes, concentrations and injection rates were not studied. The relationship of the volume of injection with respect to the size of the target vascular bed has not been established. The potential need for dose adjustment on the basis of immature renal function has not been established. In the pediatric population, the pharmacokinetic parameters have not been established.
Pediatric patients at higher risk of experiencing an adverse reaction during and after administration of any contrast agent include those with asthma, a sensitivity to medication and/or allergens, cyanotic and acyanotic heart disease, congestive heart failure, or a serum creatinine greater than 1.5 mg/dL. The injection rates in small vascular beds, and the relationship of the dose by volume or concentration in small pediatric patients have not been established. Exercise caution in selecting the dose.
Safety and effectiveness in pediatric patients below the age of two have not been established.
8.5 Geriatric Use
Middle-aged and elderly patients, without significantly impaired renal function, who received ULTRAVIST Injection in doses corresponding to 9–30 g iodine, had mean steady-state volumes of distribution that ranged between 30–40 L. Mean total and renal clearances were between 81–125 mL/min and 70–115 mL/min respectively in these patients, and were similar to the values found in the young volunteers. The distribution phase half-life in this patient population was 0.1 hour, the main elimination phase half-life was 2.3 hours, and the terminal elimination phase half-life was 40 hours. The urinary excretion (97% of the dose) and fecal excretion (2%) was comparable to that observed in young healthy volunteers, suggesting that, compared to the renal route, biliary and/or gastrointestinal excretion is not significant for iopromide.
8.6 Renal Impairment
In patients with renal impairment, opacification of the calyces and pelves by iopromide may be delayed due to slower renal excretion of iopromide.
A pharmacokinetic study in patients with mild (n=2), moderate (n=6), and severe (n=3) renal impairment was conducted. The total clearance of iopromide was decreased proportionately to the baseline decrease in creatinine clearance. The plasma AUC increased about 2-fold in patients with moderate renal impairment and 6-fold in patients with severe renal impairment compared to subjects with normal renal function. The terminal half-life increased from 2.2 hrs for subjects with normal renal function to 11.6 hrs in patients with severe renal impairment. The peak plasma concentration of iopromide was not influenced by the extent of renal impairment. Exercise caution and use the lowest necessary dose of ULTRAVIST in patients with renal dysfunction [see Warnings and Precautions (5.2)].
-
10 OVERDOSAGE
The adverse effects of overdosage are life-threatening and affect mainly the pulmonary and cardiovascular systems. Treatment of an overdosage is directed toward the support of all vital functions, and prompt institution of symptomatic therapy.
ULTRAVIST Injection binds negligibly to plasma or serum protein and can, therefore, be dialyzed.
-
11 DESCRIPTION
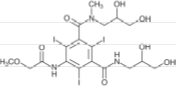
ULTRAVIST (iopromide) Injection is a nonionic, water soluble x-ray contrast agent for intravascular administration. Each Multiple-Dose container is to be used as a Pharmacy Bulk Package for dispensing multiple single dose preparations utilizing a suitable transfer device. The chemical name for iopromide is N,N'-Bis(2,3-dihydroxypropyl) –2,4,6–triiodo–5– [(methoxyacetyl)amino] –N-methyl–1,3-benzenedicarboxamide. Iopromide has a molecular weight of 791.12 (iodine content 48.12%).
Iopromide has the following structural formula:
ULTRAVIST Injection is a nonionic sterile, clear, colorless to slightly yellow, odorless, pyrogen-free aqueous solution of iopromide, containing 2.42 mg/mL tromethamine buffer and 0.1 mg/mL edetate calcium disodium stabilizer.
ULTRAVIST Injection Pharmacy Bulk Package is available in two strengths:
300 mg Iodine per mL provides 623.4 mg/mL iopromide
370 mg Iodine per mL provides 768.86 mg/mL iopromide
During the manufacture of ULTRAVIST Injection, sodium hydroxide or hydrochloric acid may be added for pH adjustment. ULTRAVIST Injection has a pH of 7.4 (6.5–8) at 25± 2°C, is sterilized by autoclaving and contains no preservatives.
The iodine concentrations (mg Iodine per mL) available have the following physicochemical properties:
ULTRAVIST
INJECTION
ULTRAVIST
INJECTION
Property
300 mg Iodine per mL
370 mg Iodine per mL
Osmolality*(mOsmol/kg water)
@ 37°C
607
774
Osmolarity*(mOsmol/L)
@ 37°C
428
496
Viscosity (cP)
@ 20°C
@ 37°C
9.2
4.9
22
10
Density (g/mL)
@ 20°C
@ 37°C
1.330
1.322
1.409
1.399
*Osmolality was measured by vapor-pressure osmometry. Osmolarity was calculated from the measured osmolal concentrations.
Solutions of ULTRAVIST Injection 300 mg Iodine per mL and 370 mg Iodine per mL have osmolalities respectively 2.1 and 2.7 times that of plasma (285 mOsmol/kg water).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Iopromide is a nonionic, water soluble, tri-iodinated x-ray contrast agent for intravascular administration.
Intravascular injection of iopromide opacifies those vessels in the path of flow of the contrast agent, permitting radiographic visualization of the internal structures until significant hemodilution occurs.
12.2 Pharmacodynamics
Following ULTRAVIST administration, the degree of contrast enhancement is directly related to the iodine content in the administered dose; peak iodine plasma levels occur immediately following rapid intravenous injection. Iodine plasma levels fall rapidly within 5 to 10 minutes. This can be accounted for by the dilution in the vascular and extravascular fluid compartments.
Intravascular Contrast: Contrast enhancement appears to be greatest immediately after bolus injections (15 seconds to 120 seconds). Thus, greatest enhancement may be detected by a series of consecutive two-to-three second scans performed within 30 to 90 seconds after injection (that is, dynamic computed tomographic imaging).
ULTRAVIST Injection may be visualized in the renal parenchyma within 30–60 seconds following rapid intravenous injection. Opacification of the calyces and pelves in patients with normal renal function becomes apparent within 1–3 minutes, with optimum contrast occurring within 5–15 minutes.
In contrast CT, some performance characteristics are different in the brain and body. In contrast CT of the body, iodinated contrast agents diffuse rapidly from the vascular into the extravascular space. Following the administration of iodinated contrast agents, the increase in tissue density to x-rays is related to blood flow, the concentration of the contrast agent, and the extraction of the contrast agent by various interstitial tissues. Contrast enhancement is thus due to any relative differences in extravascular diffusion between adjacent tissues.
In the normal brain with an intact blood-brain barrier, contrast is generally due to the presence of iodinated contrast agent within the intravascular space. The radiographic enhancement of vascular lesions, such as arteriovenous malformations and aneurysms, depends on the iodine content of the circulating blood pool.
In tissues with a break in the blood-brain barrier, contrast agent accumulates within interstitial brain tissue. The time to maximum contrast enhancement can vary from the time that peak blood iodine levels are reached to 1 hour after intravenous bolus administration. This delay suggests that radiographic contrast enhancement is at least in part dependent on the accumulation of iodine containing medium within the lesion and outside the blood pool. The mechanism by which this occurs is not clear.
For information on coagulation parameters, fibrinolysis and complement system [see Drug Interactions (7.2)].
12.3 Pharmacokinetics
Distribution
After intravenous administration to healthy young volunteers, plasma iopromide concentration time profile shows an initial distribution phase with a half-life of 0.24 hour; a main elimination phase with a half-life of 2 hours; and a terminal elimination phase with a half-life of 6.2 hours. The total volume of distribution at steady state is about 16 L suggesting distribution in to extracellular space. Plasma protein binding of iopromide is 1%.
Iodinated contrast agents may cross the blood-brain barrier [see Warnings and Precautions (5.8)].
Elimination
The amounts excreted unchanged in urine represent 97% of the dose in young healthy subjects. Only 2% of the dose is recovered in the feces. Similar recoveries in urine and feces are observed in middle-aged and elderly patients. This finding suggests that, compared to the renal route, biliary and/or gastrointestinal excretion is not important for iopromide. During the slower terminal phase only 3% of the dose is eliminated; 97% of the dose is disposed of during the earlier phases, the largest part of which occurs during the main elimination phase. The ratio of the renal clearance of iopromide to the creatinine clearance is 0.82 suggesting that iopromide is mainly excreted by glomerular filtration. Additional tubular reabsorption is possible. Pharmacokinetics of iopromide at intravenous doses up to 80 g iodine, are dose proportionate and first order.
The mean total and renal clearances are 107 mL/min and 104 mL/min, respectively.
Specific Populations
A pharmacokinetic study was conducted in 11 patients with renal impairment [see Use in Specific Populations (8.6)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed with iopromide to evaluate carcinogenic potential or effects on fertility. Iopromide was not genotoxic in a series of studies including the Ames test, an in vitro human lymphocytes analysis of chromosomal aberrations, an in vivo mouse micro-nucleus assay, and in an in vivo mouse dominant lethal assay.
-
14 CLINICAL STUDIES
ULTRAVIST Injection was administered to 708 patients; 1 patient was less than 18 years of age, 347 patients were between 18 and 59 years of age, and 360 patients were equal to or greater than 60 years of age; the mean age was 56.6 years (range 17–88). Of the 708 patients, 446 (63%) were male and 262 (37%) were female. The racial distribution was: Caucasian 463 (65.4%), Black 95 (13.4%), Hispanic 36 (5.1%), Asian 11 (1.6 %), and other or unknown 103 (14.5%). Efficacy assessment was based on the global evaluation of the quality of the radiographs by rating visualization as either excellent, good, poor, or no image, and on the ability to make a diagnosis. Five (5) intra-arterial and three (3) intravenous procedures were studied with 1 of 2 concentrations (370 mg Iodine per mL and 300 mg Iodine per mL). These procedures were: aortography/visceral angiography, coronary arteriography and left ventriculography, cerebral arteriography, peripheral arteriography, contrast computed tomography (CT) of head and body, and excretory urography.
Cerebral arteriography was evaluated in two randomized, double-blind clinical trials of ULTRAVIST Injection 300 mg Iodine per mL in 80 patients with conditions such as altered cerebrovascular perfusion and/or permeability occurring in central nervous system diseases due to various CNS disorders. Visualization ratings were good or excellent in 99% of the patients with ULTRAVIST Injection; a radiologic diagnosis was made in the majority of the patients. Confirmation of the radiologic findings by other diagnostic methods was not obtained.
Coronary arteriography/left ventriculography was evaluated in two randomized, double-blind clinical trials and one unblinded, unrandomized clinical trial of ULTRAVIST Injection 370 mg Iodine per mL in 106 patients with conditions such as altered coronary artery perfusion due to metabolic causes and in patients with conditions such as altered ventricular function. Visualization ratings were good or excellent in 99% or more of the patients a radiologic diagnosis was made in the majority of the patients. A confirmation of the radiologic findings by other diagnostic methods was not obtained.
Aortography/visceral angiography was evaluated in two randomized, double-blind clinical trials in 78 patients with conditions such as altered aortic blood flow and/or visceral vascular disorders. Visualization ratings were good or excellent in the majority of the patients; a radiologic diagnosis was made in 99% of the patients with ULTRAVIST Injection. A confirmation of radiologic findings by other diagnostic methods was not obtained. The risks of renal arteriography could not be analyzed.
Contrast CT of head and body was evaluated in three randomized, double-blind clinical trials of ULTRAVIST Injection 300 mg Iodine per mL in 95 patients with vascular disorders. Visualization ratings were good or excellent in 99% of the patients; a radiologic diagnosis was made in the majority of the patients. A confirmation of contrast CT findings by other diagnostic methods was not obtained.
ULTRAVIST Injection was evaluated in a blinded reader trial for CT of the head and body. Among the 382 patients who were evaluated with ULTRAVIST Injection 370 mg Iodine per mL, visualization ratings were good or excellent in approximately 97% of patients.
Similar studies were completed with comparable findings noted in peripheral arteriography and excretory urography.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
ULTRAVIST Injection is a sterile, clear, colorless to slightly yellow, odorless, pyrogen-free aqueous solution available in three strengths.
ULTRAVIST Injection 300 mg Iodine per mL Pharmacy Bulk Package (Multiple-Dose container)
- 200 mL fill/250 mL bottles (carton of 10) NDC: 50419-344-21
- 500 mL fill/500 mL bottles (carton of 8) NDC: 50419-344-58
ULTRAVIST Injection 370 mg Iodine per mL Pharmacy Bulk Package (Multiple-Dose container)
- 200 mL fill/250 mL bottles (carton of 10) NDC: 50419-346-26
- 500 mL fill/500 mL bottles (carton of 8) NDC: 50419-346-58
Store ULTRAVIST at 25°C (77°F); excursions permitted to 15–30°C (59–86°F) and protected from light [see USP Controlled Room Temperature].
Directions for Proper Use of ULTRAVIST Injection PHARMACY BULK PACKAGE
- 1. Perform the transfer of ULTRAVIST Injection from the PHARMACY BULK PACKAGE in a suitable work area, such as a laminar flow hood, utilizing aseptic technique.
- 2. Penetrate the container closure only one time, utilizing a suitable transfer device.
- 3. After initial puncture use the contents of the PHARMACY BULK PACKAGE within 10 hours.
- 4. Discard any unused ULTRAVIST Injection 10 hours after the initial puncture of the bulk package.
-
17 PATIENT COUNSELING INFORMATION
Instruct patients receiving ULTRAVIST Injection to inform their physician or healthcare provider of the following:
- Advise patients to inform their physician if they develop a rash after receiving ULTRAVIST [see Warnings and Precautions (5.9)]
-
Lactation: Advise lactating women that interruption of breast feeding is not necessary, however, a lactating woman may consider interrupting breastfeeding and pumping and discarding breast milk for 12 to 24 hours after ULTRAVIST Injection administration to minimize exposure to the breastfed infant (8.2)
Manufactured for:
-
Bayer HealthCare Pharmaceuticals Inc.
Whippany, NJ 07981 - Manufactured in Germany
- ©1995, Bayer HealthCare Pharmaceuticals Inc. All rights reserved.
The following are representative examples of ULTRAVIST labeling. See the "How Supplied" section for a complete listing of all components.
-
Package/Label Display Panel 240mgI/200mL
NDC: 50419-342-71 200 mL
sterile solution
pharmacy bulk package
not for direct infusion
Ultravist®
(brand of iopromide)
240 mg Iodine per mL
injection
Rx only
NOT FOR INTRATHECAL USE
Discard unused portion 10 hours after initial puncture of the container.
Dose: See package insert. For intravascular use only.
Each mL contains 498.72 mg iopromide with 2.42 mg tromethamine as a buffer and 0.1 mg edetate calcium disodium as a stabilizer.
Contains no antimicrobial preservative.
Protect from light.Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
Mfd. for:
Bayer HealthCare Pharmaceuticals Inc.
Wayne, NJ 07470
Mfd in Germany
200 mL
240
-
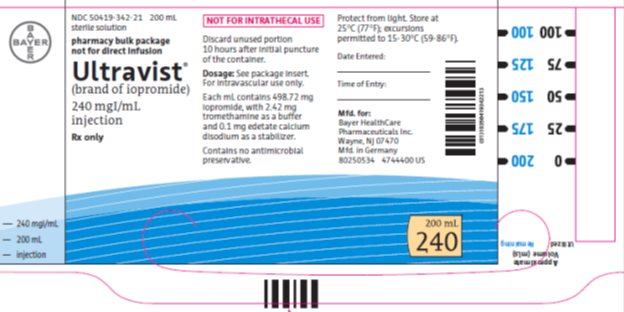
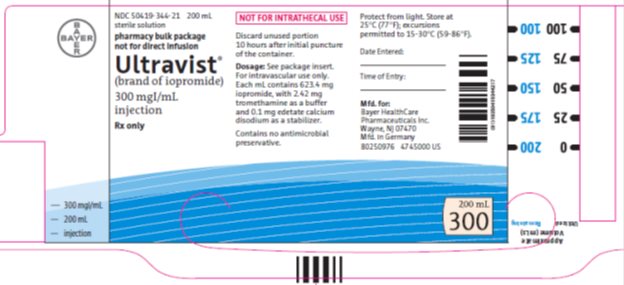
Package/Label Display Panel 300mgI/200mL
NDC: 50419-344-21 200 mL
sterile solution
pharmacy bulk package
not for direct infusion
Ultravist®
(brand of iopromide)
300 mgIodine per mL
injection
Rx only
NOT FOR INTRATHECAL USE
Discard unused portion 10 hours after initial puncture of the container.
Dose: See package insert. For intravascular use only.
Each mL contains 623.4 mg iopromide with 2.42 mg tromethamine as a buffer and 0.1 mg edetate calcium disodium as a stabilizer.
Contains no antimicrobial preservative.
Protect from light.Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
Date Entered:
Time of Entry:
Mfd. for:
Bayer HealthCare Pharmaceuticals Inc.
Wayne, NJ 07470
Mfd in Germany
200 mL
300
-
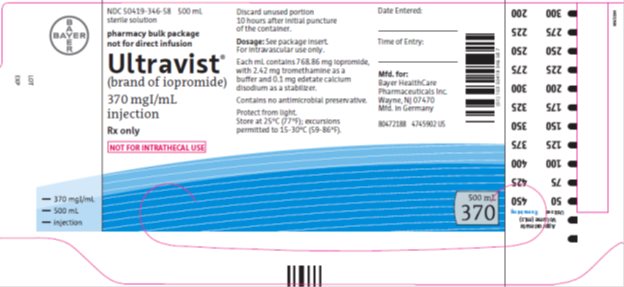
Package/Label Display Panel 370mgI/500mL
NDC: 50419-346-58 500 mL
sterile solution
pharmacy bulk package
not for direct infusion
Ultravist®
(brand of iopromide)
370 mgIodine per mL
injection
Rx only
NOT FOR INTRATHECAL USE
Discard unused portion 10 hours after initial puncture of the container.
Dose: See package insert. For intravascular use only.
Each mL contains 768.86 mg iopromide with 2.42 mg tromethamine as a buffer and 0.1 mg edetate calcium disodium as a stabilizer.
Contains no antimicrobial preservative.
Protect from light.Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
Date Entered:
Time of Entry:
Mfd. for:
Bayer HealthCare Pharmaceuticals Inc.
Wayne, NJ 07470
Mfd in Germany
500 mL
-
INGREDIENTS AND APPEARANCE
ULTRAVIST
iopromide injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50419-342 Route of Administration INTRA-ARTERIAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IOPROMIDE (UNII: 712BAC33MZ) (IOPROMIDE - UNII:712BAC33MZ) IODINE 240 mg in 1 mL Inactive Ingredients Ingredient Name Strength EDETATE CALCIUM DISODIUM (UNII: 25IH6R4SGF) TROMETHAMINE (UNII: 023C2WHX2V) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50419-342-21 10 in 1 PACKAGE 12/30/2009 04/30/2020 1 200 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021425 12/30/2009 04/30/2020 ULTRAVIST
iopromide injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50419-344 Route of Administration INTRA-ARTERIAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IOPROMIDE (UNII: 712BAC33MZ) (IOPROMIDE - UNII:712BAC33MZ) IODINE 300 mg in 1 mL Inactive Ingredients Ingredient Name Strength EDETATE CALCIUM DISODIUM (UNII: 25IH6R4SGF) TROMETHAMINE (UNII: 023C2WHX2V) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50419-344-21 10 in 1 PACKAGE 12/30/2009 1 200 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 50419-344-58 8 in 1 PACKAGE 12/30/2009 2 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC: 50419-344-48 8 in 1 PACKAGE 12/30/2009 3 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC: 50419-344-97 8 in 1 PACKAGE 10/11/2016 4 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 5 NDC: 50419-344-32 8 in 1 PACKAGE 10/11/2015 5 500 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021425 12/30/2009 ULTRAVIST
iopromide injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50419-346 Route of Administration INTRA-ARTERIAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IOPROMIDE (UNII: 712BAC33MZ) (IOPROMIDE - UNII:712BAC33MZ) IODINE 370 mg in 1 mL Inactive Ingredients Ingredient Name Strength EDETATE CALCIUM DISODIUM (UNII: 25IH6R4SGF) TROMETHAMINE (UNII: 023C2WHX2V) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50419-346-58 8 in 1 PACKAGE 12/30/2009 1 NDC: 50419-346-33 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 50419-346-26 10 in 1 PACKAGE 12/30/2009 2 NDC: 50419-346-32 200 mL in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC: 50419-346-97 8 in 1 PACKAGE 10/11/2016 3 500 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021425 12/30/2009 Labeler - Bayer HealthCare Pharmaceuticals Inc. (005436809) Establishment Name Address ID/FEI Business Operations Bayer AG 315097875 ANALYSIS(50419-342, 50419-344, 50419-346) , API MANUFACTURE(50419-342, 50419-344, 50419-346) , LABEL(50419-342, 50419-344, 50419-346) , MANUFACTURE(50419-342, 50419-344, 50419-346) , PACK(50419-342, 50419-344, 50419-346) , STERILIZE(50419-342, 50419-344, 50419-346)
Trademark Results [Ultravist]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ULTRAVIST 73708062 1542191 Live/Registered |
SCHERING AG, BERLIN AND BERGKAMEN 1988-03-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.