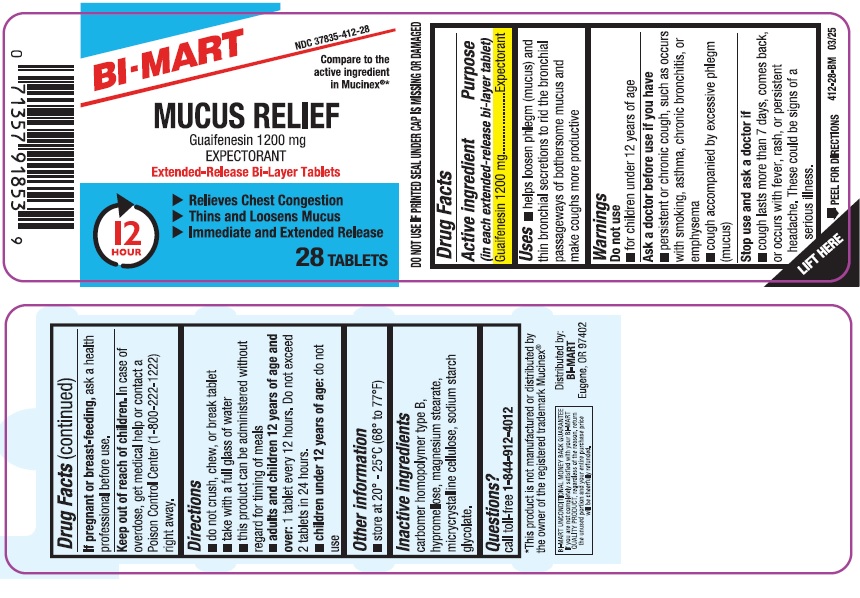
MUCUS RELIEF- guaifenesin tablet, extended release
MUCUS RELIEF by
Drug Labeling and Warnings
MUCUS RELIEF by is a Otc medication manufactured, distributed, or labeled by Bi-Mart, Ganules India Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each extended-release bi-layer tablet)
- Purpose
- Uses
- Warnings
- Ask a doctor before use if you have
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children
- Directions
- Other information
- Inactive ingredients
- Questions?
-
PRINCIPAL DISPLAY PANEL
NDC: 37835-412-28
Compare to the active ingredients in Mucinex ®*
MUCUS RELIEF
Guaifenesin 1200 mg
EXPECTORANT
Extended-Release Bi-Layer Tablets
- Relieves Chest Congestion
- Thins and Loosens Mucus
- Immediate and Extended Release
28 Tablets
*This product is not manufactured or distributed by the owner of the registered trademark Mucinex ®.
Distributed by:
BI-MART
Eugene, OR 97402
-
INGREDIENTS AND APPEARANCE
MUCUS RELIEF
guaifenesin tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 37835-412 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 1200 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSES (UNII: 3NXW29V3WO) CARBOMER HOMOPOLYMER TYPE B (ALLYL SUCROSE CROSSLINKED) (UNII: Z135WT9208) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color white Score no score Shape OVAL Size 21mm Flavor Imprint Code G;1200 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 37835-412-28 28 in 1 BOTTLE; Type 0: Not a Combination Product 04/01/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA213420 12/18/2020 Labeler - Bi-Mart (027630078) Establishment Name Address ID/FEI Business Operations Ganules India Limited 918609236 manufacture(37835-412)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.