CERETEC- technetium tc-99m exametazime and cobaltous chloride kit
Ceretec by
Drug Labeling and Warnings
Ceretec by is a Prescription medication manufactured, distributed, or labeled by Medi-Physics, Inc. dba GE Healthcare, GE Healthcare AS. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CERETEC safely and effectively. See full prescribing information for CERETEC.
CERETEC (kit for the preparation of technetium Tc 99m exametazime injection) for intravenous use.
Initial U.S. Approval: 1988INDICATIONS AND USAGE
Ceretec is a radioactive diagnostic agent, indicated in adults and pediatric patients age 2 to 17 for:
DOSAGE AND ADMINISTRATION
- Do not use cobalt stabilizer solution for leukocyte labeled scintigraphy. (2.1)
- Use appropriate radiation safety measures and aseptic technique during preparation and handling. (2.1)
- Leukocyte Labeled Scintigraphy - The recommended adult dose is 185 MBq to 370 MBq (5 mCi to 10 mCi) of Tc 99m exametazime labeled leukocytes by intravenous injection. Administer as soon as possible after labeling, preferably within 20 minutes but no later than 1 hour. (2.2)
- Cerebral Scintigraphy - The recommended adult dose is 555 MBq to 1110 MBq (15 mCi to 30 mCi) by intravenous injection. (2.2)
- See full prescribing information for preparation and administration, interpretation of chromatograms and radiation dosimetry. (2)
DOSAGE FORMS AND STRENGTHS
Each Ceretec kit consists of 5 units. (3) Each unit contains:
- One vial of Ceretec: A lyophilized mixture of 0.5 mg exametazime and 4.5 mg sodium chloride.
- One vial of cobalt stabilizer solution: 200 mcg cobalt chloride 6-hydrate stabilizer solution in 2 mL of Water for Injection.
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
Hypersensitivity reactions: Have cardiopulmonary resuscitation equipment and personnel available and monitor all patients for hypersensitivity reactions (5.1)
ADVERSE REACTIONS
Most common adverse reactions include a reversible increase in blood pressure. (6)
To report SUSPECTED ADVERSE REACTIONS, contact GE Healthcare at 1-800-654-0118 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
- Pregnancy - Advise the pregnant woman of the potential risk to the fetus based on the radiation dose from the Technetium Tc 99m exametazime injection and the gestational timing of exposure. (8.1)
- Lactation – Temporarily discontinue breastfeeding. A lactating woman should pump and discard breastmilk for 12 to 24 hours after Technetium Tc 99m Exametazime labeled leukocyte administration. (8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Leukocyte Labeled Scintigraphy
1.2 Cerebral Scintigraphy
2 DOSAGE AND ADMINISTRATION
2.1 Important Preparation and Radiation Safety Instructions
2.2 Recommended Dosing and Imaging Procedures
2.3 Preparation and Administration Instructions
2.4 Preparation of Autologous Leukocytes
2.5 Preparation of Tc 99m Exametazime Injection with Cobalt Stabilizer Solution
2.6 Preparation of Technetium Tc 99m Exametazime Injection Without Cobalt Stabilizer Solution
2.7 Radiation Dosimetry
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Risk for Image Interpretation Errors
5.3 Radiation Exposure Risk
6 ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
11 DESCRIPTION
11.1 Chemical Characteristics
11.2 Physical Characteristics
11.3 External Radiation
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Leukocyte Labeled Scintigraphy
Ceretec, when reconstituted with technetium Tc 99m exametazime (without cobalt stabilizer solution), is indicated in adults and pediatric patients age 2 to 17 for leukocyte labeled scintigraphy as an adjunct in the localization of intraabdominal infection and inflammatory bowel disease.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Preparation and Radiation Safety Instructions
- The Ceretec kit includes a cobalt stabilizer solution, which is optional for cerebral scintigraphy. DO NOT USE COBALT STABILIZER SOLUTION FOR LEUKOCYTE LABELED Scintigraphy [see Dosage and Administration (2.4, 2.6)].
- Technetium Tc 99m exametazime injection is a radioactive drug and should be handled with appropriate safety measures to minimize radiation exposure [see Warnings and Precautions (5.3)]. Use waterproof gloves and effective shielding, including syringe shields, when preparing and administering technetium Tc 99m exametazime injection.
2.2 Recommended Dosing and Imaging Procedures
Leukocyte Labeled Scintigraphy
Dosing
Imaging Procedures
- Instruct patients to empty their bladder prior to imaging.
- Dynamic imaging may be performed for the first 60 minutes after injection to assess lung clearance and to visualize cell migration.
- Perform static imaging at 0.5-1.5 hours, 2-4 hours and if necessary, at 18-24 hours after administration to detect focal accumulation of activity.
2.3 Preparation and Administration Instructions
General Preparation and Administration Instructions
- Use aseptic procedures throughout preparation and handling.
- Visually inspect the reconstituted technetium Tc 99m exametazime injection prior to use and do not use if there is evidence of particulate matter or discoloration.
- Measure patient dose with a dose calibrator immediately prior to administration.
- Instruct patients to maintain adequate hydration, after administration of technetium Tc 99m exametazime labeled white blood cells or Tc 99m exametazime injection and void frequently to minimize radiation dose to the kidneys and bladder [see Warnings and Precautions (5.3)].
Reconstitution Instructions
- Elute the technetium Tc 99m generator according to the manufacturer's instructions.
- Only use eluate from a technetium Tc 99m generator which has been eluted within the previous 24 hours.
- For the highest radiochemical purity reconstitute with freshly eluted technetium 99m generator eluate.
- To prepare technetium Tc 99m exametazime injection for white blood cell labeling, use generator elute that is not more than 2 hours old.
- To prepare technetium Tc 99m exametazime injection with cobalt stabilizer for cerebral imaging, use generator eluate that is not more than 4 hours old.
2.4 Preparation of Autologous Leukocytes
Leukocyte Harvest and Separation
1) Draw up 10 mL acid citrate dextrose solution into a 60 mL syringe.
2) Withdraw approximately 40 mL whole blood from the patient into the syringe using a 19-gauge butterfly needle infusion set. Close the syringe with a sterile hub.
3) Gently mix the contents of the syringe for 20 seconds.
4) Clamp the syringe barrel to the ring stand in an upright (hub side up) position and tilt the syringe 10-20 degrees from its position perpendicular to the bench.
5) Allow the red cells to sediment 30-60 minutes, until the supernatant [leukocyte rich plasma (LRP)] looks clear of red blood cells.
6) Using an infusion set, transfer the leukocyte-rich plasma (LRP), the supernatant, from the previous step, into a sterile, conical centrifuge tube marked "WBC" (white blood cell) and assure that only a minimum amount of red cells enter the centrifuge tube.
7) Immediately centrifuge the capped WBC tube at 400-450 g for 5 minutes. The plasma will separate out into a liquid [leukocyte poor plasma (LPP)] and a solid (WBC button). (Note: The button often contains a small number of red cells and may appear red).
8) Transfer the supernatant into another sterile tube marked "LPP" leaving enough supernatant to cover the white cell button.
Reserve LPP for later use (steps 11,15,18).
Washing and Radiolabeling
9) Add approximately 5 mL Sodium Chloride Injection, USP (0.9%) to the WBC button. Cap the "WBC" tube and resuspend the button by gently swirling.
10) Centrifuge the capped "WBC" tube at 150 g for 8 minutes and discard all but 0.5 to 1 mL of the supernatant to cover the cells.
11) Add 1 mL of "LPP" (from Step 9) to the white cell button and resuspend the cells by gentle swirling.
12) Reconstitute technetium Tc 99m exametazime from Ceretec with generator eluate [see Dosage and Administration (2.6)].
13) Within 30 minutes of preparation, add the reconstituted Tc 99m exametazime (do not use cobalt stabilizer solution) to the "WBC" tube. Swirl gently to mix.
14) Set a lab timer for 15 minutes and allow the white cells to incubate. Swirl at 30 second intervals during the incubation.
15) After incubation, add 10 mL of the LPP (from Step 9) to the "WBC" tube.
16) Cap the "WBC" tube, gently swirl, and then centrifuge at 450 g for 5 minutes.
17) Transfer the supernatant in the "WBC" tube into the "Wash" tube and leave the labeled white cells in the "WBC" tube.
18) Add approximately 5 mL of LPP (from Step 9) to the "WBC" tube. Gently swirl to resuspend the cells.
19) Draw the labeled cells into a syringe. Cap the syringe and assay the amount of radioactivity in a dose calibrator. Place the syringe in a shielded container.
20) Verify the identity of the labeled leukocyte recipient.
21) Administer the Tc 99m labeled leukocyte suspension using a 19G needle as soon as possible, preferably within 1-2 hours after labeling.
2.5 Preparation of Tc 99m Exametazime Injection with Cobalt Stabilizer Solution
- The preparation may be used in cerebral scintigraphy
- 1) Add up to 370 MBq to 2000 MBq (10 mCi to 54 mCi) sodium pertechnetate Tc 99m eluate to the shielded Ceretec vial.
- 2) Before reconstitution, the technetium Tc 99m generator eluate may be adjusted to the correct radioactive concentration [74 to 400 MBq/mL (2 to 10.8 mCi/mL)] by dilution with a volume of 5 mL preservative-free, non-bacteriostatic 0.9% sodium chloride for injection.
- 3) Between 1 and 5 minutes after reconstitution, inject 2 mL of cobalt stabilizer solution into the vial. Shake the shielded vial for 10 seconds to ensure complete mixing.
- 4) The cobalt stabilized technetium 99m exametazime is a pale straw-colored solution and the pH is in the range 5 to 8.
- 5) Use a Sample for Radiochemical Purity measurement.
- 6) Assay the vial for total radioactivity. Calculate the volume to be injected. Complete the label provided and attach to the vial.
- 7) Use the stabilized product within 5 hours after preparation. Individual patient doses may be stored aseptically in a capped syringe if required.
- 8) Discard any unused material.
Radiochemical Purity Measurement Tc 99m Exametazime Injection with Cobalt Stabilizer Solution
Obtain the Following Materials:
- Two GMCP-SA (Glass Microfiber Chromatography Paper impregnated with Silicic Acid) strips (2 cm (±2 mm) × 20 cm)
- Ascending chromatography development tanks
- MEK [methyl ethyl ketone (butanone)]
- 0.9 % sodium chloride
A combination of two chromatographic systems is necessary for the determination of the radiochemical purity of the injection:
- System 1 GMCP-SA: MEK [methyl ethyl ketone (butanone)]
- System 2 GMCP-SA:0.9% sodium chloride
Three potential radiochemical impurities may be present in prepared Technetium (99mTc) Exametazime Injection
- secondary Tc 99m exametazime complex
- free Tc 99m pertechnetate
- reduced-hydrolyzed Tc 99m
Method
- 1) Perform radiochemical purity testing as soon as possible after preparation.
- 2) Prepare the two Chromatographic Systems (System 1 and System 2).
- 3) Apply test samples by needle approximately 2.5 cm from the bottom of each GMCP-SA strip.
- 4) Immediately place each strip in prepared ascending chromatography development tanks. After the solvent has travelled to the 14 cm mark, remove the strips and mark the solvent fronts.
- 5) Allow the strips to dry.
- 6) Determine the distribution of activity determined using suitable equipment.
- 7) Chromatogram Interpretation:
System 1 (GMCP-SA: MEK [butanone]) Origin Secondary Tc 99m exametazime complex and reduced-hydrolyzed Tc 99m Migrate at Rf 0.8-1 Lipophilic Tc 99m exametazime complex and Tc 99m pertechnetate System 2 (GMCP-SA:0.9% sodium chloride) Origin Lipophilic Tc 99m exametazime complex, secondary Tc 99m exametazime complex, and reduced-hydrolyzed Tc 99m Migrate at Rf 0.8-1 Tc 99m pertechnetate - 8) Calculate the percentage of activity due to both secondary Tc 99m exametazime complex and reduced-hydrolyzed Tc 99m from System 1 (A %).
- 9) Calculate the percentage of activity due to Tc 99m pertechnetate from System 2 (B %).
- 10)
Calculate the radiochemical purity:
% lipophilic Tc 99m exametazime complex = 100 - (A %+B %)- A % represents the level of secondary Tc 99m exametazime complex plus reduced-hydrolyzed Tc 99m.
- B % represents the level of Tc 99m pertechnetate.
- 11) Do not use if radiochemical purity of lipophilic Tc 99m exametazime is less than 80%.
2.6 Preparation of Technetium Tc 99m Exametazime Injection Without Cobalt Stabilizer Solution
The preparation may be used in cerebral scintigraphy or for use in the preparation of Tc 99m labeled leukocytes.
- 1) Add 370 MBq to 2000 MBq (10 mCi up to 54 mCi) of sodium pertechnetate Tc 99m eluate.
- 2) Before reconstitution, the technetium Tc 99m generator eluate may be adjusted to the correct radioactive concentration [74 to 400 MBq/mL (2 to 10.8 mCi/mL)] by dilution with a volume of 5 mL preservative-free, non-bacteriostatic 0.9 % sodium chloride for injection.
- 3) The pH of the prepared injection is 9 to 9.8.
- 4) Use a sample for Radiochemical Purity Measurement.
- 5) Assay the total activity.
- 6) Calculate the volume to be injected and complete the label provided and attach to the vial shield.
- 7) Use the preparation within 30 minutes after reconstitution.
- 8) Discard any unused material.
Radiochemical Purity Measurement Tc 99m Exametazime Injection without Cobalt Stabilizer Solution
- Perform radiochemical purity testing of technetium Tc 99m exametazime within 2 minutes of reconstitution.
- The entire procedure takes approximately 15 minutes.
Obtain the Following Materials:
- 2 SA ITLC strips 20 cm × 2 cm
- 1 Whatman No. 1 strips 6 cm × 0.7 cm
- MEK (methyl ethyl ketone [butanone])
- 0.9% aqueous sodium chloride (non-bacteriostatic)
- 50% aqueous acetonitrile
- Dilute with non-bacteriostatic Water for Injection
- Glass test tubes (12 × 75 mm)
- Glass measuring cylinders (100 mL) with covers
- 1 mL syringes with 25-gauge needles
- A combination of 3 chromatographic systems is necessary for the complete definition of the radiochemical composition of the injection.
- System 1: MEK (methyl ethyl ketone [butanone]) + SA ITLC strip
- System 2: 0.9% non-bacteriostatic sodium chloride solution + SA ITLC strip
- System 3: 50% acetonitrile solution + Whatman No. 1 paper strip
- Three potential radiochemical impurities may be present in the prepared injection of the lipophilic Tc 99m exametazime complex.
- a secondary Tc 99m exametazime complex
- free Tc 99m pertechnetate
- reduced-hydrolyzed Tc 99m
Method
1) Prepare chromatography tubes (Identify the solvent in each cylinder).
System 1 - 100 mL cylinder containing a 1 cm depth of fresh MEK.
System 2 - 100 mL cylinder containing a 1 cm depth of 0.9% sodium chloride.
System 3 - 1 chromatography tube containing 0.2-0.3 mL of 50% acetonitrile, respectively.2) Prepare 2 SA ITLC strips and 1 Whatman No. 1 paper strip.
Mark the SA ITLC strips 2.5 cm from the bottom as the point of origin.
Mark both the SA ITLC strips at 14 cm above the origin (solvent front).
Mark the Whatman strip 1 cm from the bottom as the point of origin.3) Apply at least 5 microliter samples of freshly prepared Tc 99m exametazime solution to the origin of the 3 strips (within 2 minutes of reconstitution). Do not allow to dry.
4) Immediately place 1 SA ITLC strip into the MEK tank (System 1), the second SA ITLC strip into the saline tank (System 2), and the Whatman No. 1 paper strip into the 50% acetonitrile tube (System 3).
5) The SA ITLC MEK strip takes approximately 15 minutes to run. When the eluate has reached the solvent front remove the strip from the tube with forceps and immediately cut 1 cm above the origin.
6) The SA ITLC saline strip takes approximately 15 minutes to run. When the eluate has reached the solvent front remove the strip from the tube with forceps and immediately cut 2.5 cm above the origin.
7) The Whatman No. 1 paper CH3CN strip takes approximately 100 seconds to run. When the eluate has reached the solvent front mark remove the strip from the tube with forceps and immediately cut 0.5 cm above the origin.
8) Chromatogram Interpretation:
System 1 (SA ITLC: MEK (methyl ethyl ketone [butanone]) Origin Secondary Tc 99m exametazime complex and reduced-hydrolyzed Tc 99m Migrate at Rf 0.8-1 Lipophilic Tc 99m exametazime complex and Tc 99m pertechnetate System 2 (SA ITLC: 0.9% sodium chloride) Origin Lipophilic Tc 99m exametazime complex, secondary Tc 99m, exametazime complex and reduced-hydrolyzed Tc 99m Migrate at Rf 0.8-1 Tc 99m pertechnetate System 3 (Whatman No. 1: 50% aqueous acetonitrile) Origin Reduced-hydrolyzed Tc 99m Migrate at Rf 0.8-1 Lipophilic Tc 99m exametazime complex, secondary Tc 99m exametazime complex and Tc 99m pertechnetate 9) Count the separate sections of each strip to determine the activity distribution. Calculate:
- % origin of saline strip (system 2)
- % origin of MEK strip (system 1)
- % solvent front of saline strip (= % Tc 99m pertechnetate)
- % origin of Whatman No. 1 paper strip (= % reduced-hydrolyzed Tc 99m)
10) Calculate Radiochemical Purity
% lipophilic Tc 99m exametazime complex = % origin of saline strip (system 2) – % origin of MEK strip (system 1)
11) Do not use if the radiochemical purity of the lipophilic Tc 99m exametazime complex is less than 80%.
2.7 Radiation Dosimetry
Tc 99m Exametazime labeled leukocytes for leukocyte labeled scintigraphy
Radiation absorbed dose per unit activity (microGy/MBq) administered to average-size adults (70 kg) and pediatric patients from an intravenous injection of Tc 99m Exametazime labeled leukocytes is estimated in Table 1.
Table 1: Estimated Radiation Absorbed Dose for Tc 99m exametazime labeled white blood cells (leukocytes) Organ Absorbed dose per unit activity administered (microGy / MBq) Adult 15 years 10 years 5 years 1 year *International Commission on Radiological Protection, Radiation Dose to Patients from Radiopharmaceuticals: A Compendium of Current Information Related to Frequently Used Substances, Ann ICRP 2015)., ICRP Publication 128, Ann ICRP 2015). Adrenals 12 12 18 26 43 Bone surfaces 16 21 34 61 150 Brain 2.3 2.9 4.4 7 13 Breast 2.4 2.9 4.9 7.6 13 Gallbladder wall 8.4 10 16 25 36 Gastrointestinal tract - Esophagus
3.5 4.2 5.8 8.6 15 - Stomach wall
8.1 9.6 14 20 32 - Small intestine wall
4.6 5.7 8.7 13 21 - Colon wall
4.3 5.4 8.4 12 21 - Upper large intestine wall
4.7 5.9 9.3 14 23 - Lower large intestine wall
3.7 4.8 7.3 10 18 Heart wall 9.4 12 17 25 44 Kidneys 12 14 22 32 54 Liver 20 26 38 54 97 Lungs 7.8 9.9 15 23 41 Muscles 3.3 4.1 6 8.9 16 Ovaries 3.9 5 7.2 11 18 Pancreas 13 16 23 34 53 Red marrow 23 25 40 71 140 Skin 1.8 2.1 3.4 5.5 10 Spleen 150 210 310 480 850 Testes 1.6 2.1 3.2 5.1 9.2 Thymus 3.5 4.2 5.8 8.6 15 Thyroid 2.9 3.7 5.8 9.3 17 Urinary bladder wall 2.6 3.5 5.2 7.8 14 Uterus 3.4 4.3 6.5 9.7 16 Remaining organs 3.4 4.2 6.3 9.5 16 Effective dose per unit activity 11 microSv/MBq 14 microSv/MBq 22 microSv/MBq 34 microSv/MBq 62 microSv/MBq Tc 99m Exametazime Injection for Cerebral Scintigraphy
Based on human data, the radiation absorbed doses to average sized adults (70kg) and pediatric patients from an intravenous injection of Tc 99m exametazime injection are estimated in Table 2.
Table 2: Estimated Radiation Absorbed Dose for Tc 99m Exametazime Injection Organ Absorbed dose per unit activity administered (microGy / MBq) Adult 15 years 10 years 5 years 1 year *International Commission on Radiological Protection, Radiation Dose to Patients from Radiopharmaceuticals: A Compendium of Current Information Related to Frequently Used Substances, Ann ICRP 2015)., ICRP Publication 128, Ann ICRP 2015). Adrenals 5.3 6.7 9.9 14 24 Bone surfaces 5.1 6.4 9.4 14 24 Brain 6.8 11 16 21 37 Breast 2 2.4 3.7 5.6 9.5 Gallbladder wall 18 21 28 48 140 Gastrointestinal tract - Esophagus
2.6 3.3 4.7 6.9 11 - Stomach wall
6.4 8.5 12 19 36 - Small intestine wall
12 15 24 36 65 - Colon wall
17 22 35 55 100 - Upper large intestine wall
18 24 38 60 110 - Lower large intestine wall
15 19 31 48 90 Heart wall 3.7 4.7 6.7 9.7 16 Kidneys 34 41 57 81 140 Liver 8.6 11 16 23 40 Lungs 11 16 22 34 63 Muscles 2.8 3.5 5 7.3 13 Ovaries 6.6 8.3 12 17 27 Pancreas 5.1 6.5 9.7 14 23 Red marrow 3.4 4.1 5.9 8 14 Skin 1.6 1.9 2.9 4.5 8.3 Spleen 4.3 5.4 8.2 12 20 Testes 2.4 3 4.4 6.1 11 Thymus 2.6 3.3 4.7 6.9 11 Thyroid 26 42 63 140 260 Urinary bladder wall 23 28 33 33 56 Uterus 6.6 8.1 12 15 25 Remaining organs 3.2 4 6 9.2 17 Effective dose per unit activity 9.3 microSv/MBq 11 microSv/MBq 17 microSv/MBq 27 microSv/MBq 49 microSv/MBq The effective dose resulting from the administration of a (maximal recommended) activity of 1110 MBq for an adult weighing 70 kg is about 10.3 mSv. For an administered activity of 740 MBq the typical radiation dose to the target organ (brain) is 5 mGy and the typical radiation dose to the critical organ (kidneys) is 25 mGy.
-
3 DOSAGE FORMS AND STRENGTHS
The Ceretec Kit is supplied as a five-unit package. Each unit contains:
- One (10 mL, multiple-dose) vial of Ceretec: A lyophilized mixture of 0.5 mg exametazime, sealed under nitrogen atmosphere with a rubber closure.
- One vial cobalt stabilizer solution: 200 mcg cobaltous chloride 6-hydrate stabilizer solution in 2 mL of Water for Injection.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions have been reported following the administration of Tc 99m exametazime injection and Tc 99m exametazime labeled leukocytes including serious signs and symptoms of anaphylaxis following administration [see Adverse Reactions (6)]. Always have cardiopulmonary resuscitation equipment and personnel available and monitor all patients for hypersensitivity reactions.
5.2 Risk for Image Interpretation Errors
The interpretation of images for leukocyte labeled imaging can be affected by the presence of other pathophysiological processes such as: tumor, infarction, trauma, and inflammatory conditions.
5.3 Radiation Exposure Risk
Technetium Tc 99m contributes to a patient's overall long-term cumulative radiation exposure, which is associated with an increased risk of cancer. Ensure safe handling and preparation procedures to protect patients and health care workers from unintentional radiation exposure. Encourage patients to drink fluids and instruct patients to void when the examination is completed and as often thereafter as frequently as possible after administration [see Dosage and Administration (2.1, 2.3)]. Radiation risks associated with the use of Tc 99m exametazime injection and Tc 99m exametazime labeled leukocytes are greater in pediatric patients than in adults due to greater radiosensitivity and longer life expectancy.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)].
The following adverse reactions associated with the use of Ceretec were identified in clinical studies or postmarketing reports. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular disorders: Reversible increase in blood pressure, flushing
Gastrointestinal disorders: Nausea, vomiting
General disorders and administration site conditions: Malaise, fatigue, fever
Immune system disorders: Hypersensitivity reactions: anaphylactic reactions including shock, facial edema, rash, pruritus or erythema
Nervous system disorders: Headache, dizziness, paresthesia
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited available data with technetium Tc 99m exametazime use in pregnant women are insufficient to inform any drug associated risks for major birth defects and miscarriage. Technetium Tc 99m exametazime is transferred across the placenta [see Data]. Animal reproduction studies with technetium Tc 99m exametazime have not been conducted. However, all radiopharmaceuticals have the potential to cause fetal harm depending on the fetal stage of development and the magnitude of the radiation dose. If considering technetium Tc 99m exametazime administration to a pregnant woman, inform the patient about the potential for adverse pregnancy outcomes based on the radiation dose from technetium Tc 99m exametazime and the gestational timing of exposure.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Human Data
Limited published literature describes Tc 99m exametazime crossing the placental barrier and visualization of radioactivity in the fetal liver. No adverse fetal effects or radiation-related risks have been identified for diagnostic procedures involving less than 50 mGy, which represents less than 10 mGy fetal doses.
8.2 Lactation
Risk Summary
There are limited data available in the scientific literature on the presence of technetium Tc 99m exametazime in human milk. There are no data available regarding the effects of technetium Tc 99m exametazime on the breastfed infant or on milk production. Exposure of technetium Tc 99m exametazime to a breast fed infant can be minimized by temporary discontinuation of breastfeeding [see Clinical Considerations]. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Ceretec and any potential adverse effects on the breastfed child from Ceretec or from the underlying maternal condition.
Clinical Considerations
To decrease radiation exposure to the breastfed infant, advise a lactating woman to pump and discard breast milk after the administration of technetium Tc 99m exametazime injection or technetium Tc 99m exametazime-labeled leukocytes for 12 to 24 hours, where the duration corresponds to the typical range of administered activity, 259 MBq to 925 MBq (7 mCi to 25 mCi).
8.4 Pediatric Use
Ceretec is indicated for use in pediatric patients from 2 to 17 years of age for leukocyte labelled scintigraphy and brain scintigraphy. The use of Ceretec for leukocyte labelled scintigraphy and brain scintigraphy is supported by extrapolation from clinical effectiveness in adults. The safety and dosing recommendations are based on clinical experience.
Safety and effectiveness in pediatric patients less than 2 years of age have not been established.
8.5 Geriatric Use
Clinical studies of Ceretec did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
-
11 DESCRIPTION
11.1 Chemical Characteristics
Ceretec (kit for the preparation of technetium Tc 99m exametazime injection) prepares a radioactive diagnostic agent for intravenous use.
Each unit consists of the following:
- One 10 mL vial of exametazime containing a sterile, non-pyrogenic, lyophilized mixture of 0.5 mg exametazime, 7.6 mcg stannous chloride dihydrate (minimum stannous tin 0.6 mcg; maximum total stannous and stannic tin 4 mcg per vial) and 4.5 mg sodium chloride, sealed under nitrogen atmosphere with a rubber closure. The product contains no antimicrobial preservative.
- One vial of cobalt stabilizer containing a sterile, non-pyrogenic solution of 200 mcg cobaltous chloride 6-hydrate in 2 mL of Water for Injection.
The chemical name for Exametazime is [(RR,SS)-4.8-diaza-3,6,6,9-tetramethylundecane-2, 10-dione bisoxime]. The molecular formula of exametazime is C13H28N4O2, with the following structural formula:
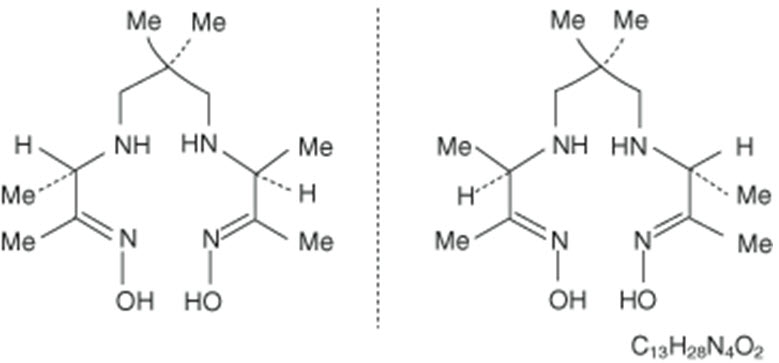
Prior to publication of the USAN, exametazime was known as hexamethylpropylene amine oxime (HM-PAO). The name HM-PAO appears in many publications.
When sterile pyrogen-free sodium pertechnetate Tc 99m in isotonic saline is added to the vial of exametazime, a Tc 99m complex of exametazime is formed.
11.2 Physical Characteristics
Technetium Tc 99m decays by isomeric transition with a physical half-life of 6 hours. Photons that are useful for imaging studies are listed in Table 3.
Table 3: Principal Radiation Emission Data-technetium Tc 99m Radiation Mean % Disintegration Mean Energy
(keV)Gamma 2 88.5 140.5 11.3 External Radiation
The air-kerma-rate (exposure-rate) constant for technetium Tc 99m is 5.23 m2∙pGy∙(MBq)–1∙s–1 [0.795 cm2∙R∙(mCi)–1∙h–1]. The first half-value thickness of lead (Pb) for technetium Tc 99m is 0.25 mm. A range of values for the relative attenuation of the radiation emitted by this radionuclide that results from interposition of various thicknesses of Pb is shown in Table 4. For example, the use of a 3 mm thickness of Pb will decrease the external radiation exposure by a factor of approximately 1,000.
Table 4: Radiation Attenuation by Lead Shielding Shield Thickness
(Pb) mmCoefficient of Attenuation 0.25 0.5 1 10–1 2 10–2 3 10–3 4 10–4 5 10–5 To correct for physical decay of this radionuclide, the fractions that remain at selected intervals relative to the time of calibration are shown in Table 5.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
General
When technetium Tc 99m pertechnetate is added to exametazime in the presence of stannous reductant, a lipophilic technetium Tc 99m complex is formed. This lipophilic complex is the active moiety and is able to cross the blood-brain barrier as well as penetrate cell membranes to label leukocytes. It converts to a polar hydrophilic species at approximately 12% per hour. The polar hydrophilic species is unable to cross the blood-brain barrier or cell membranes.
Mechanism for Labeling Leukocytes
When incubated with leukocytes, which have been isolated from whole blood, the technetium Tc 99m exametazime complex, being lipid-soluble, penetrates the cell membrane of the leukocytes by passive diffusion. The lipophilic complex is then converted to a polar hydrophilic species, in a process possibly involving intracellular glutathione, thus trapping the technetium Tc 99m label within the cells.
Mechanism for Brain Uptake
The agent localizes in the brain as a function of regional cerebral perfusion. Technetium Tc 99m exametazime is primarily extracted and trapped by cerebral gray matter and the basal ganglia during the first pass through the brain. It has been proposed that the retention in the brain of technetium Tc 99m exametazime results from in vivo conversion of the primary complex to a less lipophilic complex, which is unable to cross the blood-brain barrier.
12.2 Pharmacodynamics
The relationship between Tc 99m exametazime plasma concentrations and successful imaging is not known.
12.3 Pharmacokinetics
Distribution
Leukocytes Labeled with Tc 99m Exametazime
During the first hour following injection of Tc 99m labeled leukocytes, activity is seen in the lungs, liver, spleen, blood pool, bone marrow, kidneys, gall bladder, and urinary bladder. At 4 hours following injection, lung radioactivity was decreased and bone marrow radioactivity was increased.
The lipophilic Tc 99m exametazime complex (that is not stabilized by cobalt chloride) is taken up by leukocytes and selectively retained in neutrophils. Label elution rate is up to 10% in the first hour.
Tc 99m Exametazime Injection
Studies in normal volunteers have shown that the technetium Tc 99m exametazime is rapidly cleared from the blood after intravenous injection. Uptake in the brain reaches a maximum of 3.5 to 7% of the injected dose within one minute of injection. Up to 15% of the activity is eliminated from the brain by 2 minutes post injection, after which little activity is lost for the following 24 hours except by physical decay of technetium Tc 99m.
Elimination
Excretion
Leukocytes Labeled with Tc 99m Exametazime
Following injection of Tc 99m labeled leukocytes, over the first 1 to 6 hours, the Tc 99m is visualized in the bowel. At 24 hours post-injection substantial colonic activity is seen, implying that excretion of radioactivity takes place through bowel.
Tc 99m Exametazime Injection
About 30% of the injected dose is found in the gastrointestinal tract immediately after injection and about 50% of this is excreted through the intestinal tract over 48 hours. About 40% of the injected dose is excreted through the kidneys and urine over the 48 hours after injection.
The use of cobalt stabilizer solution for stabilization prior to injection does not appear to affect the pharmacokinetic handling or distribution of Tc 99m exametazime.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Ceretec without cobalt stabilizer solution
Long term animal studies have not been performed to evaluate carcinogenic potential or whether exametazime affects fertility in males or females. When evaluated in the Ames test, exametazime increased the apparent rate of gene mutation in the TA100 strain of S. typhimurium. Exametazime did not cause chromosomal aberrations in vitro (Chinese Hamster Ovary cells) or in vivo (rat bone marrow).
Ceretec with cobalt stabilizer solution
In-vitro mutagenicity studies indicate that the stabilized formulation of technetium-99m exametazime is weakly mutagenic in the Ames (bacterial mutation) test, human lymphocyte chromosome aberration assay and mouse lymphoma thymidine kinase assay.
The stabilized formulation is not mutagenic in two in-vivo assays (rat bone marrow micronucleus and rat liver micronucleus).
-
14 CLINICAL STUDIES
Two clinical trials were performed in a total of 88 patients who had suspected intra-abdominal infection or inflammation. Subjects received both Tc 99m labeled leukocytes and a radiolabeled comparator. Images were obtained at 2 and 30 minutes and at 2 and 4 hours and 24 hours. In two other clinical trials, in a total of 127 patients with suspected abdominal inflammation or infection received Tc 99m labeled leukocytes. Imaging was at 24 hours in one study and at 1, 3 and 24 hours in the other. In all four studies images were blindly evaluated and the findings were confirmed by surgery, biopsy or other clinical data.
Based on the above 4 studies, between 2 to 4 hours Tc 99m labeled leukocytes had 95-100% sensitivity and 62-85% specificity. In all studies, the false positive and false negatives relate to the bowel background, the location of the site of infection/inflammation and whether or not it is contiguous with the bowel. Images obtained at 24 hours can be unreliable because of a high bowel background; false negatives were noted in both Tc 99m and the radiolabeled comparator.
Other studies suggest that the interpretation of the images could be affected by the presence of tumors, infarction and peritonitis; liver abscess may be missed because of the bowel background.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How supplied
Each Ceretec Kit (NDC: 17156-025-05) contains:
- Five 10mL vials of 0.5 mg exametazime
- Five 10mL vials of 200 mcg Cobalt stabilizer solution
- Ten radiation labels
- One package insert
Sodium Pertechnetate Tc 99m is not part of the Ceretec kit. Before reconstitution and radiolabeling with Tc 99m, the contents of the kit are not radioactive.
16.2 Storage and Handling
Store Ceretec kits at controlled room temperature 15°C to 25°C (59°F to 77°F).
This reagent kit is approved for use by persons licensed by the U.S. Nuclear Regulatory Commission or the relevant Agreement State. Store and dispose of technetium Tc 99m exametazime in compliance with the regulations of the government agency authorized to license the use of this radionuclide.
-
17 PATIENT COUNSELING INFORMATION
Administration Instructions
Instruct patients to remain hydrated and void frequently following administration to decrease radiation exposure [see Dosage and Administration (2.3)].
Pregnancy
Advise pregnant women of the risk of fetal exposure to radiation doses if they undergo a radionuclide imaging study [see Use in Specific Populations (8.1)].
Lactation
Advise a lactating woman to pump and discard breast milk for 12-24 hours (based on injection dose) after administration to minimize radiation exposure to the breastfed infant [see Use in Specific Populations (8.2)].
-
SPL UNCLASSIFIED SECTION
Distributed by:
GE Healthcare
Medi-Physics, Inc.
Arlington Heights, IL 60004Manufactured by:
GE Healthcare AS
Oslo, NorwayProduct of Norwegian Origin.
Ceretec is a trademark of GE Healthcare.
GE and the GE Monogram are trademarks of General Electric Company.
© 2018 General Electric Company - All rights reserved.
Patent No. 4,789,736
-
PRINCIPAL DISPLAY PANEL - 5 mL Vial Label
GE Healthcare
CERETEC™
Technetium Tc99m Exametazime InjectionNDC: 17156-022-05
Rx ONLY
NOT FOR RESALEFor the preparation of Technetium Tc99m Exametazime Injection
Vial contains in lyophilized form 0.5 mg exametazime, 7.6 mcg
stannous chloride dihydrate (minimum stannous tin 0.6 mcg;
maximum total stannous and stannic tin 4 mcg per vial) and 4.5
mg sodium chloride. Sealed under nitrogen. Store the kit at
15°-25°C (59°-77°F). For intravenous injection after
reconstitution with Technetium Tc99m.
Not for use in humans until Technetium Tc99m is added as
directed in accompanying package insert. Contains no
antimicrobial preservative. Store the reconstituted drug at
20°-25°C (68°-77°F) using appropriate radiation shielding
and use within time specified in the package insert for
the appropriate injection preparation. Do not freeze.Mfd. for: GE Healthcare, Medi-Physics, Inc.,
Arlington Hts., IL 60004, 1-800-292-8514
By: GE Healthcare AS, Oslo, Norway
40-9159H-OSLO1194438 USA
EXP
LOT
-
PRINCIPAL DISPLAY PANEL - 2.5 mL Vial Label
GE Healthcare
Cobalt (II)
chloride 6-hydrateNDC: 17156-027-01
Rx ONLY
NOT FOR
RESALECobalt stabilizer solution
For use only with reconstituted Ceretec™.
Not for direct administration.Vial contains sterile, non-pyrogenic cobalt stabilizer solution
of 200 mcg cobalt chloride 6-hydrate stabilizer solution
in 2 mL of Water for Injection. Use as directed in package
insert. Store at 15°-25°C (59°-77°F). Do not freeze.Manufactured for: GE Healthcare
Medi-Physics, Inc.
Arlington Heights, IL 60004, 1-800-292-8514
By: GE Healthcare AS, Oslo, Norway1193054 USA
Exp.:
Lot:

-
PRINCIPAL DISPLAY PANEL - Kit Carton Label
GE Healthcare
CERETEC™
Technetium Tc99m Exametazime InjectionNDC: 17156-025-05
Five-unit package
Storage: Store the kit at 15°-25°C (59°-77°F).
After reconstitution with Technetium Tc99m,
store at 20°-25°C (68°-77°F). Do not freeze.Use appropriate radiation shielding.
Not for use in humans until technetium
Tc99m is added.For intravenous use as directed.
For Dosage and Administration:
See Package Insert.Contents: Each package contains the following:
Five Ceretec vials. Each vial contains a lyophilized form of 0.5 mg exametazime.
7.6 mcg, stannous chloride dihydrate (minimum stannous tin
0.6 mcg, maximum total stannous and stannic tin 4 mcg per vial) and
4.5 mg sodium chloride.Five Cobalt (II) chloride 6-hydrate vials. Each vial contains
sterile, non-pyrogenic cobalt stabilizer solution of 200 mcg
cobalt chloride 6-hydrate stabilizer solution in 2 mL of Water for Injection.Ten radiation labels
One package insert
For preparation of Technetium Tc99m Exametazime
Injection see package insert.
-
INGREDIENTS AND APPEARANCE
CERETEC
technetium tc-99m exametazime and cobaltous chloride kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 17156-025 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17156-025-05 1 in 1 CARTON 09/25/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 5 VIAL, GLASS 25 mL Part 2 5 VIAL, GLASS 12.5 mL Part 1 of 2 CERETEC
technetium tc-99m exametazime injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 17156-022 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Technetium Tc-99m Exametazime (UNII: 3B744AG22N) (Technetium Tc-99m Exametazime - UNII:3B744AG22N) EXAMETAZIME 0.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength Stannous Chloride (UNII: 1BQV3749L5) 7.6 ug in 5 mL Sodium Chloride (UNII: 451W47IQ8X) 4.5 mg in 5 mL Nitrogen (UNII: N762921K75) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17156-022-05 5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019829 12/30/1988 Part 2 of 2 COBALT CHLORIDE
cobaltous chloride injection, solutionProduct Information Item Code (Source) NDC: 17156-027 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Cobaltous Chloride (UNII: EVS87XF13W) (COBALTOUS CATION - UNII:AI1MR454XG) Cobaltous Chloride 200 ug in 2 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17156-027-01 2.5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019829 09/25/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019829 09/25/2018 Labeler - Medi-Physics, Inc. dba GE Healthcare (095263729) Establishment Name Address ID/FEI Business Operations GE Healthcare AS 515048908 MANUFACTURE(17156-025) , RELABEL(17156-025) , REPACK(17156-025)
Trademark Results [Ceretec]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CERETEC 73715173 1645766 Live/Registered |
AMERSHAM INTERNATIONAL PLC 1988-03-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.