KINERET- anakinra injection, solution
Kineret by
Drug Labeling and Warnings
Kineret by is a Prescription medication manufactured, distributed, or labeled by Swedish Orphan Biovitrum AB (publ), Boehringer Ingelheim Pharma GmbH & Co KG (BIP, an affiliate to BI RCV), Germany, Boehringer Ingelheim RCV, Austria, Charles River Laboratories Edinburgh Ltd., UK, Charles River Laboratories Inc. USA , Eurofins Biolab S.r.l., Italy, Hospira Zagreb d.o.o., Croatia, Patheon Italia S.p.A., Italy, Pfizer Health AB, Sweden, Rechon Life Science AB, Sweden, Swedish Orphan Biovitrum AB (publ), Sweden. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use KINERET safely and effectively. See full prescribing information for KINERET.
KINERET® (anakinra) injection, for subcutaneous use
Initial U.S. Approval: 2001INDICATIONS AND USAGE
Kineret is an interleukin-1 receptor antagonist indicated for:
Rheumatoid Arthritis (RA)
- Reduction in signs and symptoms and slowing the progression of structural damage in moderately to severely active rheumatoid arthritis, in patients 18 years of age or older who have failed 1 or more disease modifying antirheumatic drugs (DMARDs) (1.1)
Cryopyrin-Associated Periodic Syndromes (CAPS)
- Treatment of Neonatal-Onset Multisystem Inflammatory Disease (NOMID) (1.2)
DOSAGE AND ADMINISTRATION
Rheumatoid Arthritis (RA)
- The recommended dose of Kineret for the treatment of patients with rheumatoid arthritis is 100 mg/day administered daily by subcutaneous injection. The dose should be administered at approximately the same time every day (2.1)
- Physicians should consider a dose of 100 mg of Kineret administered every other day for RA patients who have severe renal insufficiency or end stage renal disease (defined as creatinine clearance < 30 mL/min, as estimated from serum creatinine levels) (2.3)
Cryopyrin-Associated Periodic Syndromes (CAPS)
- The recommended starting dose of Kineret is 1-2 mg/kg daily for NOMID patients. The dose can be individually adjusted to a maximum of 8 mg/kg daily to control active inflammation. (2.2)
- Physicians should consider administration of the prescribed Kineret dose every other day for NOMID patients who have severe renal insufficiency or end stage renal disease (defined as creatinine clearance < 30 mL/min, as estimated from serum creatinine levels) (2.3)
See full prescribing information for administration instructions (2.4)
DOSAGE FORMS AND STRENGTHS
Injection: 100 mg/0.67 mL solution in a single-use prefilled syringe for subcutaneous injection. Graduated syringe allows for doses between 20 mg and 100 mg. (3)
CONTRAINDICATIONS
- Known hypersensitivity to E coli-derived proteins, Kineret, or to any component of the product. (4)
WARNINGS AND PRECAUTIONS
- In RA, discontinue use if serious infection develops. In Kineret-treated NOMID patients, the risk of a NOMID flare when discontinuing Kineret treatment should be weighed against the potential risk of continued treatment. Do not initiate Kineret in patients with active infections. (5.1)
- Use in combination with Tumor Necrosis Factor (TNF) blocking agents is not recommended (5.2)
- Hypersensitivity reactions, including anaphylactic reactions and angioedema, have been reported (5.3)
- The impact of treatment with Kineret on active and/or chronic infections and the development of malignancies is not known (5.4)
- Live vaccines should not be given concurrently with Kineret (5.5)
- Neutrophil counts should be assessed prior to initiating Kineret treatment, and while receiving Kineret, monthly for 3 months, and thereafter quarterly for a period up to 1 year (5.6)
ADVERSE REACTIONS
Rheumatoid Arthritis (RA)
Most common adverse reactions (incidence ≥ 5%) are injection site reaction, worsening of rheumatoid arthritis, upper respiratory tract infection, headache, nausea, diarrhea, sinusitis, arthralgia, flu like-symptoms, and abdominal pain (6.1)
NOMID
The most common AEs during the first 6 months of treatment (incidence >10%) are injection site reaction, headache, vomiting, arthralgia, pyrexia, and nasopharyngitis (6.2)
To report SUSPECTED ADVERSE REACTIONS, contact 1-866-547-0644 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- A higher rate of serious infections has been observed in RA patients treated with concurrent Kineret and etanercept therapy than in patients treated with etanercept alone. Use of Kineret in combination with TNF blocking agents is not recommended (7)
USE IN SPECIFIC POPULATIONS
- Pediatric use: Kineret is indicated for use in pediatric patients with NOMID (8.4)
- Geriatric use: Because there is a higher incidence of infections in the elderly population in general, caution should be used in treating the elderly (8.5)
- Renal impairment: This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function (8.6)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Active Rheumatoid Arthritis
1.2 Cryopyrin-Associated Periodic Syndromes (CAPS)
2 DOSAGE AND ADMINISTRATION
2.1 Active Rheumatoid Arthritis
2.2 Cryopyrin-Associated Periodic Syndromes (CAPS)
2.3 Renal Impairment
2.4 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious Infections
5.2 Use With TNF Blocking Agents
5.3 Hypersensitivity Reactions
5.4 Immunosuppression
5.5 Immunizations
5.6 Neutrophil Count
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience in RA
6.2 Clinical Study Experience in NOMID
6.3 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 TNF Blocking Agents
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
14 CLINICAL STUDIES
14.1 Clinical Studies in RA
14.2 Clinical Studies in NOMID
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Active Rheumatoid Arthritis
Kineret is indicated for the reduction in signs and symptoms and slowing the progression of structural damage in moderately to severely active rheumatoid arthritis (RA), in patients 18 years of age or older who have failed 1 or more disease modifying antirheumatic drugs (DMARDs). Kineret can be used alone or in combination with DMARDs other than Tumor Necrosis Factor (TNF) blocking agents [see Warnings and Precautions (5.2)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Active Rheumatoid Arthritis
The recommended dose of Kineret for the treatment of patients with rheumatoid arthritis is 100 mg/day administered daily by subcutaneous injection. Higher doses did not result in a higher response. The dose should be administered at approximately the same time every day.
2.2 Cryopyrin-Associated Periodic Syndromes (CAPS)
The recommended starting dose of Kineret is 1-2 mg/kg for NOMID patients. The dose can be individually adjusted to a maximum of 8 mg/kg daily to control active inflammation.
Adjust doses in 0.5 to 1.0 mg/kg increments. Once daily administration is generally recommended, but the dose may be split into twice daily administrations. Each syringe is intended for a single use. A new syringe must be used for each dose. Any unused portion after each dose should be discarded.
2.3 Renal Impairment
Physicians should consider administration of the prescribed dose of Kineret every other day for patients who have severe renal insufficiency or end stage renal disease (defined as creatinine clearance < 30 mL/min, as estimated from serum creatinine levels) [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
2.4 Administration
Instructions on appropriate use should be given by the healthcare provider to the patient or caregiver. Patients or caregivers should not be allowed to administer Kineret until the patient or caregiver has demonstrated a thorough understanding of procedures and an ability to inject the product correctly. The prescribed dose of Kineret should be administered according to the instructions for use and any unused portions discarded. After administration of Kineret it is essential to follow the proper procedure for disposal of syringes and any residual drug. See the “Information for Patients” insert for detailed instructions on the handling and injection of Kineret.
Do not use Kineret beyond the expiration date shown on the carton. Visually inspect the solution for particulate matter and discoloration before administration. There may be trace amounts of small, translucent-to-white amorphous particles of protein in the solution. The prefilled syringe should not be used if the solution is discolored or cloudy, or if foreign particulate matter is present. If the number of translucent-to-white amorphous particles in a given syringe appears excessive, do not use this syringe.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Kineret is contraindicated in patients with known hypersensitivity to E coli-derived proteins, Kineret, or any components of the product [see Hypersensitivity Reactions (5.3)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Serious Infections
Kineret has been associated with an increased incidence of serious infections (2%) vs. Placebo (< 1%) in clinical trials in RA. Administration of Kineret in RA should be discontinued if a patient develops a serious infection. In Kineret treated NOMID patients the risk of a NOMID flare when discontinuing Kineret treatment should be weighed against the potential risk of continued treatment. Treatment with Kineret should not be initiated in patients with active infections. The safety and efficacy of Kineret in immunosuppressed patients or in patients with chronic infections have not been evaluated.
Drugs that affect the immune system by blocking tumor necrosis factor (TNF) have been associated with an increased risk of reactivation of latent tuberculosis (TB). It is possible that taking drugs such as Kineret that blocks IL-1 increases the risk of TB or other atypical or opportunistic infections. Health care providers should follow current CDC guidelines both to evaluate for and to treat possible latent tuberculosis infections before initiating therapy with Kineret.
5.2 Use With TNF Blocking Agents
In a 24-week study of concurrent Kineret and etanercept therapy in RA patients, the rate of serious infections in the combination arm (7%) was higher than with etanercept alone (0%). The combination of Kineret and etanercept did not result in higher ACR response rates compared to etanercept alone [see clinical studies (14)]. Use of Kineret in combination with TNF blocking agents is not recommended.
5.3 Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylactic reactions and angioedema, have been reported with Kineret. If a severe hypersensitivity reaction occurs, administration of Kineret should be discontinued and appropriate therapy initiated.
5.4 Immunosuppression
The impact of treatment with Kineret on active and/or chronic infections and the development of malignancies is not known [see Adverse Reactions (6)].
5.5 Immunizations
In a placebo-controlled clinical trial (n = 126), no difference was detected in anti-tetanus antibody response between the Kineret and placebo treatment groups when the tetanus/diphtheria toxoids vaccine was administered concurrently with Kineret. No data are available on the effects of vaccination with other inactivated antigens in patients receiving Kineret. No data are available on either the effects of live vaccination or the secondary transmission of infection by live vaccines in patients receiving Kineret. Therefore, live vaccines should not be given concurrently with Kineret.
5.6 Neutrophil Count
Patients receiving Kineret may experience a decrease in neutrophil counts. Neutrophil counts should therefore be assessed prior to initiating Kineret treatment, and while receiving Kineret, monthly for 3 months, and thereafter quarterly for a period up to 1 year.
In the placebo-controlled studies, 8% of RA patients receiving Kineret had decreases in neutrophil counts of at least one World Health Organization (WHO) toxicity grade compared with 2% in the placebo control group. Nine Kineret-treated patients (0.4%) experienced neutropenia (ANC < 1 x 109/L). This is discussed in more detail in the Adverse Reactions (6): Hematologic Events (6.1) section.
In 43 NOMID patients followed for up to 60 months 2 patients experienced neutropenia that resolved over time during continued Kineret treatment. [see Adverse Reactions (6.2)]
-
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
6.1 Clinical Studies Experience in RA
The most serious adverse reactions were:
- Serious Infections – [see Warnings and Precautions (5.1)]
- Neutropenia, particularly when used in combination with TNF blocking agents
The most common adverse reaction with Kineret is injection-site reactions. These reactions were the most common reason for withdrawing from studies.
The data described herein reflect exposure to Kineret in 3025 patients, including 2124 exposed for at least 6 months and 884 exposed for at least one year. Studies 1 and 4 used the recommended dose of 100 mg per day. The patients studied were representative of the general population of patients with rheumatoid arthritis.
Injection-site Reactions
The most common and consistently reported treatment-related adverse event associated with Kineret is injection-site reaction (ISR). In Studies 1 and 4, 71% of patients developed an ISR, which was typically reported within the first 4 weeks of therapy. The majority of ISRs were reported as mild (72.6% mild, 24.1% moderate and 3.2% severe). The ISRs typically lasted for 14 to 28 days and were characterized by 1 or more of the following: erythema, ecchymosis, inflammation, and pain.
Infections
In Studies 1 and 4 combined, the incidence of infection was 39% in the Kineret-treated patients and 37% in placebo-treated patients during the first 6 months of blinded treatment. The incidence of serious infections in Studies 1 and 4 was 2% in Kineret-treated patients and 1% in patients receiving placebo over 6 months. The incidence of serious infection over 1 year was 3% in Kineret-treated patients and 2% in patients receiving placebo. These infections consisted primarily of bacterial events such as cellulitis, pneumonia, and bone and joint infections. Majority of patients (73%) continued on study drug after the infection resolved. No serious opportunistic infections were reported. Patients with asthma appeared to be at higher risk of developing serious infections when treated with Kineret (8 of 177 patients, 4.5%) compared to placebo (0 of 50 patients, 0%).
In open-label extension studies, the overall rate of serious infections was stable over time and comparable to that observed in controlled trials. In clinical studies and postmarketing experience, cases of opportunistic infections have been observed and included fungal, mycobacterial and bacterial pathogens. Infections have been noted in all organ systems and have been reported in patients receiving Kineret alone or in combination with immunosuppressive agents.
In patients who received both Kineret and etanercept for up to 24 weeks, the incidence of serious infections was 7%. The most common infections consisted of bacterial pneumonia (4 cases) and cellulitis (4 cases). One patient with pulmonary fibrosis and pneumonia died due to respiratory failure.
Malignancies
Among 5300 RA patients treated with Kineret in clinical trials for a mean of 15 months (approximately 6400 patient years of treatment), 8 lymphomas were observed for a rate of 0.12 cases/100 patient years. This is 3.6 fold higher than the rate of lymphomas expected in the general population, based on the National Cancer Institute's Surveillance, Epidemiology and End Results (SEER) database.3 An increased rate of lymphoma, up to several fold, has been reported in the RA population, and may be further increased in patients with more severe disease activity. Thirty-seven malignancies other than lymphoma were observed. Of these, the most common were breast, respiratory system, and digestive system. There were 3 melanomas observed in Study 4 and its long-term open-label extension, greater than the 1 expected case. The significance of this finding is not known. While patients with RA, particularly those with highly active disease, may be at a higher risk (up to several fold) for the development of lymphoma, the role of IL-1 blockers in the development of malignancy is not known.
Hematologic Events
In placebo-controlled studies with Kineret, 8% of patients receiving Kineret had decreases in total white blood counts of at least one WHO toxicity grade, compared with 2% of placebo patients. Nine Kineret-treated patients (0.4%) developed neutropenia (ANC < 1 x 109/L). 9 % of patients receiving Kineret had increases in eosinophil differential percentage of at least one WHO toxicity grade, compared with 3 % of placebo patients. Of patients treated concurrently with Kineret and etanercept 2% developed neutropenia (ANC < 1 x 109/L). While neutropenic, one patient developed cellulitis which recovered with antibiotic therapy. 2% of patients receiving Kineret had decreases in platelets, all of WHO toxicity grade one, compared to 0% of placebo patients.
Hypersensitivity Reactions
Hypersensitivity reactions including anaphylactic reactions, angioedema, urticaria, rash, and pruritus have been reported with Kineret.
Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. In Studies 1 and 4, from which data is available for up to 36 months, 49% of patients tested positive for anti-anakinra binding antibodies at one or more time points using a biosensor assay. Of the 1615 patients with available data at Week 12 or later, 30 (2%) tested positive for neutralizing antibodies in a cell-based bioassay. Of the 13 patients with available follow-up data, 5 patients remained positive for neutralizing antibodies at the end of the studies. No correlation between antibody development and adverse events was observed.
The detection of antibody formation is highly dependent on the sensitivity and specificity of the assays. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including sample handling, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to Kineret with the incidence of antibodies to other products may be misleading.
Lipids
Cholesterol elevations were observed in some patients treated with Kineret.
Other Adverse Events
Table 1 reflects adverse events in Studies 1 and 4, that occurred with a frequency of ≥ 5% in Kineret-treated patients over a 6-month period.
Table 1: Percent of RA Patients Reporting Adverse Events (Studies 1 and 4) Placebo Kineret
100 mg/dayPreferred term (n = 733) (n = 1565) Injection Site Reaction 29% 71% Worsening of RA 29% 19% Upper Respiratory Tract Infections 17% 14% Headache 9% 12% Nausea 7% 8% Diarrhea 5% 7% Sinusitis 7% 7% Arthralgia 6% 6% Flu Like Symptoms 6% 6% Abdominal Pain 5% 5% 6.2 Clinical Study Experience in NOMID
The data described herein reflect an open-label study in 43 NOMID patients exposed to Kineret for up to 60 months adding up to a total exposure of 159.8 patient years.
Patients were treated with a starting dose of 1 to 2 mg/kg/day and an average maintenance dose of 3-4 mg/kg/day adjusted depending on the severity of disease. Among pediatric NOMID patients, doses up to 7.6 mg/kg/day have been maintained for up to 15 months.
There were 24 serious adverse events (SAEs) reported in 14 of the 43 treated patients. The most common type of SAEs reported were infections [see Warnings and Precautions (5.1)]. Five SAEs were related to lumbar puncture, which was part of the study procedure.
There were no permanent discontinuations of study drug treatment due to AEs. Doses were adjusted in 5 patients because of AEs; all were dose increases in connection with disease flares.
The reporting frequency of AEs was highest during the first 6 months of treatment. The incidence of AEs did not increase over time, and no new types of AEs emerged.
The most commonly reported AEs during the first 6 months of treatment (incidence >10%) were injection site reaction (ISR), headache, vomiting, arthralgia, pyrexia, and nasopharyngitis (Table 2).
The most commonly reported AEs during the 60-month study period, calculated as the number of events/patient years of exposure, were arthralgia, headache, pyrexia, upper respiratory tract infection, nasopharyngitis, and rash.
The AE profiles for different age groups <2 years, 2-11 years, and 12-17 years corresponded to the AE profile for patients ≥18 years, with the exception of infections and related symptoms being more frequent in patients <2 years.
Infections
The reporting rate for infections was higher during the first 6 months of treatment (2.3 infections/patient-year) compared to after the first 6 months (1.7 infections/patient year). The most common infections were upper respiratory tract infection, sinusitis, ear infections, and nasopharyngitis.
There were no deaths or permanent treatment discontinuations due to infections. In one patient Kineret administration was temporarily stopped during an infection and in 5 patients the dose of Kineret was increased due to disease flares in connection with infections. Thirteen infections in 7 patients were classified as serious, the most common being pneumonia and gastroenteritis occurring in 3 and 2 patients, respectively. No serious opportunistic infections were reported.
The reporting frequency for infections was highest in patients <12 years of age.
Hematologic Events
After start of Kineret treatment neutropenia was reported in 2 patients. One of these patients experienced an upper respiratory tract infection and an otitis media infection. Both episodes of neutropenia resolved over time with continued Kineret treatment.
Injection Site Reactions
In total, 17 injection site reactions (ISRs) were reported in 10 patients during the 60-month study period. Out of the 17 ISRs, 11 (65%) occurred during the first month and 13 (76%) were reported during the first 6 months. No ISR was reported after Year 2 of treatment. The majority of ISRs were reported as mild (76% mild, 24% moderate). No patient permanently or temporarily discontinued Kineret treatment due to injection site reactions.
Immunogenicity
The immunogenicity of Kineret in NOMID patients was not evaluated.
Table 2. Most common (>10% of patients) treatment-emergent adverse events during the first 6 months of Kineret treatment Safety population (N=43) Total exposure in patient years= 20.8 Preferred term N (%) Number of events /patient year Injection site reaction 7 (16.3%) 0.5 Headache 6 (14.0%) 0.7 Vomiting 6 (14.0%) 0.6 Arthralgia 5 (11.6%) 0.6 Pyrexia 5 (11.6%) 0.4 Nasopharyngitis 5 (11.6%) 0.3 The most common adverse reactions occurring after the first 6-month period of treatment with Kineret (up to 60 months of treatment) included: arthralgia, headache, pyrexia, upper respiratory tract infection, nasopharyngitis, and rash.
6.3 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of Kineret. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hepato-biliary disorders:
- elevations of transaminases,
- non-infectious hepatitis
Hematologic events:
- thrombocytopenia, including severe thrombocytopenia (i.e platelet counts <10x109/L)
-
7 DRUG INTERACTIONS
No drug-drug interaction studies in human subjects have been conducted. Toxicologic and toxicokinetic studies in rats did not demonstrate any alterations in the clearance or toxicologic profile of either methotrexate or Kineret when the two agents were administered together.
7.1 TNF Blocking Agents
A higher rate of serious infections has been observed in patients treated with concurrent Kineret and etanercept therapy than in patients treated with etanercept alone [see Warnings and Precautions (5.2)]. Two percent of patients treated concurrently with Kineret and etanercept developed neutropenia (ANC < 1 x 109/L). Use of Kineret in combination with TNF blocking agents is not recommended.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from retrospective studies and case reports on KINERET use in pregnant women are insufficient to identify a drug associated risk of major birth defects, miscarriage, or maternal and fetal adverse events (see Data). There are risks to the mother and fetus associated with active rheumatoid arthritis or Cryopyrin-Associated Periodic Syndromes (CAPS) (see Clinical Considerations). In animal reproduction studies, subcutaneous administration of anakinra to pregnant rats and rabbits during organogenesis demonstrated no evidence of fetal harm at doses up to 25 times the maximum recommended human dose (MRHD) (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Published data suggest the risk of adverse pregnancy outcomes in women with rheumatoid arthritis or CAPS is associated with increased disease activity. Adverse pregnancy outcomes include preterm delivery (before 37 weeks of gestation), low birth weight (<2500 grams), and small for gestational age at birth.
Data
Human Data
The available data from retrospective studies and case reports of anakinra-exposed pregnancies have not identified an increased frequency or pattern of birth defects, miscarriage, or adverse maternal or fetal outcomes. An international multi-center retrospective study of pregnancy outcomes with interleukin-1 inhibitors reported on 23 anakinra-exposed pregnancies. There were 21 live births of healthy infants, 1 miscarriage, and 1 infant with left renal agenesis. The estimated background rate of detected renal malformations is 0.2-2% of all newborns. Another retrospective study reported on 10 anakinra-exposed pregnancies in women with CAPS. There were 9 live births, 1 miscarriage, and 1 fetal demise in a twin pregnancy. The surviving twin was born healthy. Overall, these data cannot definitively establish or exclude any anakinra-associated risks during pregnancy. Methodological limitations of these data include small sample size and the inability to control for confounders such as the timing of drug exposure, underlying maternal disease, and concomitant medication use.
Animal Data
Animal reproduction studies were conducted in rats and rabbits. In embryo-fetal development studies, anakinra was administered throughout the period of organogenesis at the subcutaneous doses of 12.5, 50, and 200 mg/kg/day to pregnant rats from gestation days (GD) 7 to 17 and pregnant rabbits from GD 6 to 18. In these studies, anakinra at doses up to 25 times the MRHD (on a mg/kg basis at maternal subcutaneous doses up to 200 mg/kg/day) revealed no evidence of harm to the fetus.
8.2 Lactation
Risk Summary
There are no data on the presence of anakinra in either human or animal milk or the effects on milk production. Available published data from a small retrospective study and postmarketing case reports do not establish an association between maternal anakinra use during lactation and adverse effects on breastfed infants. The limited clinical data during lactation precludes a clear determination of the risk of KINERET to an infant during lactation. Therefore, the developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for KINERET and any potential adverse effects on the breastfed infant from KINERET or from the underlying maternal condition.
8.4 Pediatric Use
The NOMID study included 36 pediatric patients: 13 below 2 years, 18 between 2 and 11 years, and 5 between 12 and 17 years of age. A subcutaneous Kineret starting dose of 1–2 mg/kg/day was administered in all age groups. An average maintenance dose of 3–4 mg/kg/day was adequate to maintain clinical response throughout the study irrespective of age but a higher dose was, on occasion, required in severely affected patients. The prefilled syringe does not allow doses lower than 20 mg to be administered.
Kineret was studied in a single randomized, blinded multi-center trial in 86 patients with polyarticular course Juvenile Rheumatoid Arthritis (JRA; ages 2-17 years) receiving a dose of 1 mg/kg subcutaneously daily, up to a maximum dose of 100 mg. The 50 patients who achieved a clinical response after a 12-week open-label run-in were randomized to Kineret (25 patients) or placebo (25 patients), administered daily for an additional 16 weeks. A subset of these patients continued open-label treatment with Kineret for up to 1 year in a companion extension study. An adverse event profile similar to that seen in adult RA patients was observed in these studies. These study data are insufficient to demonstrate efficacy and, therefore, Kineret is not recommended for pediatric use in Juvenile Rheumatoid Arthritis.
8.5 Geriatric Use
A total of 752 RA patients ≥ 65 years of age, including 163 patients ≥ 75 years of age, were studied in clinical trials. No differences in safety or effectiveness were observed between these patients and younger patients, but greater sensitivity of some older individuals cannot be ruled out. Because there is a higher incidence of infections in the elderly population in general, caution should be used in treating the elderly.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function.
8.6 Renal Impairment
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function [see Clinical Pharmacology (12.3)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
Kineret (anakinra) is a recombinant, nonglycosylated form of the human interleukin-1 receptor antagonist (IL-1Ra). Kineret differs from native human IL-1Ra in that it has the addition of a single methionine residue at its amino terminus. Kineret consists of 153 amino acids and has a molecular weight of 17.3 kilodaltons. It is produced by recombinant DNA technology using an E coli bacterial expression system.
Kineret is supplied in single use prefilled glass syringes with 29 gauge needles as a sterile, clear, colorless-to-white, preservative free solution for daily subcutaneous (SC) administration. The solution may contain trace amounts of small, translucent-to-white amorphous proteinaceous particles. Each prefilled glass syringe contains: 0.67 mL (100 mg) of anakinra in a solution (pH 6.5) containing anhydrous citric acid (1.29 mg), disodium EDTA (0.12 mg), polysorbate 80 (0.70 mg), and sodium chloride (5.48 mg) in Water for Injection, USP.
The prefilled syringe contains an outer rigid plastic needle shield attached to an inner needle cover. The syringe or needle shield components are not made with natural rubber latex.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Kineret blocks the biologic activity of IL-1 alpha and beta by competitively inhibiting IL-1 binding to the interleukin-1 type I receptor (IL-1RI), which is expressed in a wide variety of tissues and organs.
IL-1 production is induced in response to inflammatory stimuli and mediates various physiologic responses including inflammatory and immunological responses. IL-1 has a broad range of activities including cartilage degradation by its induction of the rapid loss of proteoglycans, as well as stimulation of bone resorption. The levels of the naturally occurring IL-1Ra in synovium and synovial fluid from RA patients are not sufficient to compete with the elevated amount of locally produced IL-1.
Spontaneous mutations in the CIAS1/NLRP3 gene have been identified in a majority of patients with cryopyrin-associated periodic syndromes such as NOMID. CIAS1/NLRP3 encodes for cryopyrin, a component of the inflammasome. The activated inflammasome results in proteolytic maturation and secretion of IL-1β, which has an important role in the systemic inflammation and manifestations of NOMID.
12.3 Pharmacokinetics
The absolute bioavailability of Kineret after a 70 mg subcutaneous bolus injection in healthy subjects (n = 11) is 95%. In subjects with RA, maximum plasma concentrations of Kineret occurred 3 to 7 hours after subcutaneous administration of Kineret at clinically relevant doses (1 to 2 mg/kg; n = 18); the terminal half-life ranged from 4 to 6 hours. In RA patients, no unexpected accumulation of Kineret was observed after daily subcutaneous doses for up to 24 weeks.
The influence of demographic covariates on the pharmacokinetics of Kineret was studied using population pharmacokinetic analysis encompassing 341 patients receiving daily subcutaneous injection of Kineret at doses of 30, 75, and 150 mg for up to 24 weeks. The estimated Kineret clearance increased with increasing creatinine clearance and body weight. After adjusting for creatinine clearance and body weight, gender and age were not significant factors for mean plasma clearance.
In NOMID patients, at a median SC dose of 3 mg/kg once daily and a median treatment time of 3.5 years, the median (range) steady-state serum exposure of anakinra was Cmax 3628 (655–8511) ng/mL (n=16) and C24h 203 (53–1979) ng/mL (n=16). The median (range) half-life of anakinra was 5.7 (3.1–28.2) hours (n=12). There was no obvious gender difference.
Patients With Renal Impairment: The mean plasma clearance of Kineret in subjects with mild (creatinine clearance 50-80 mL/min) and moderate (creatinine clearance 30-49 mL/min) renal insufficiency was reduced by 16% and 50%, respectively. In severe renal insufficiency and end stage renal disease (creatinine clearance < 30 mL/min1), mean plasma clearance declined by 70% and 75%, respectively. Less than 2.5% of the administered dose of Kineret was removed by hemodialysis or continuous ambulatory peritoneal dialysis. Based on these observations, a dose schedule change should be considered for subjects with severe renal insufficiency or end stage renal disease [see Dosage and Administration (2.2)].
Patients with Hepatic Dysfunction: No formal studies have been conducted examining the pharmacokinetics of Kineret administered subcutaneously in patients with hepatic impairment.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
Long-term animal studies to evaluate the carcinogenic potential of Kineret were not conducted. Kineret had no effects on fertility and reproductive performance indices in male and female rats subcutaneous doses up to 200 mg/kg/day (approximately 25 times theMRHD on a mg/kg basis).
-
14 CLINICAL STUDIES
14.1 Clinical Studies in RA
The safety and efficacy of Kineret have been evaluated in three randomized, double-blind, placebo-controlled trials of 1790 patients ≥ 18 years of age with active rheumatoid arthritis (RA). An additional fourth study was conducted to assess safety. In the efficacy trials, Kineret was studied in combination with other disease-modifying antirheumatic drugs (DMARDs) other than Tumor Necrosis Factor (TNF) blocking agents (Studies 1 and 2) or as a monotherapy (Study 3).
Study 1 involved 899 patients with active RA who had been on a stable dose of methotrexate (MTX) (10 to 25 mg/week) for at least 8 weeks. All patients had at least 6 swollen/painful and 9 tender joints and either a C-reactive protein (CRP) of ≥ 1.5 mg/dL or an erythrocyte sedimentation rate (ESR) of 28 mm/hr. Patients were randomized to Kineret or placebo in addition to their stable doses of MTX. The first 501 patients were evaluated for signs and symptoms of active RA. The total 899 patients were evaluated for progression of structural damage.
Study 2 evaluated 419 patients with active RA who had received MTX for at least 6 months including a stable dose (15 to 25 mg/week) for at least 3 consecutive months prior to enrollment. Patients were randomized to receive placebo or one of five doses of Kineret subcutaneous daily for 12 to 24 weeks in addition to their stable doses of MTX.
Study 3 evaluated 472 patients with active RA and had similar inclusion criteria to Study 1 except that these patients had received no DMARD for the previous 6 weeks or during the study. Patients were randomized to receive either Kineret or placebo. Patients were DMARD-naïve or had failed no more than 3 DMARDs.
Study 4 was a placebo-controlled, randomized trial designed to assess the safety of Kineret in 1414 patients receiving a variety of concurrent medications for their RA including some DMARD therapies, as well as patients who were DMARD-free. The TNF blocking agents etanercept and infliximab were specifically excluded. Concurrent DMARDs included MTX, sulfasalazine, hydroxychloroquine, gold, penicillamine, leflunomide, and azathioprine. Unlike Studies 1, 2 and 3, patients predisposed to infection due to a history of underlying disease such as pneumonia, asthma, controlled diabetes, and chronic obstructive pulmonary disease (COPD) were also enrolled [see Adverse Reactions (6)].
In Studies 1, 2 and 3, the improvement in signs and symptoms of RA was assessed using the American College of Rheumatology (ACR) response criteria (ACR20, ACR50, ACR70). In these studies, patients treated with Kineret were more likely to achieve an ACR20 or higher magnitude of response (ACR50 and ACR70) than patients treated with placebo (Table 3). The treatment response rates did not differ based on gender or ethnic group. The results of the ACR component scores in Study 1 are shown in Table 4.
Most clinical responses, both in patients receiving placebo and patients receiving Kineret, occurred within 12 weeks of enrollment.
Table 3: Percent of Patients with ACR Responses in Studies 1 and 3 a p < 0.05, Kineret versus placebo
b p < 0.01, Kineret versus placebo
c p < 0.001, Kineret versus placebo
Study 1 (Patients on MTX) Study 3 (No DMARDs) Kineret
ResponsePlacebo (n = 251) Kineret 100 mg/day
(n = 250)Placebo (n = 119) 75 mg/day
(n = 115)150 mg/day
(n = 115)ACR20
Month 3
Month 624%
22%
34%a 38%c 23%
27%33%
34%33%
43%aACR50
Month 3
Month 6
6%
8%13%b 17%b 5%
8%10%
11%8%
19%aACR70
Month 3
Month 60%
2%3%a 6%a 0%
1%0%
1%0%
1%Table 4: Median ACR Component Scores in Study 1 a Health Assessment Questionnaire; 0 = best, 3 = worst; includes eight categories: dressing and grooming, arising, eating, walking, hygiene, reach, grip, and activities.
b Visual analog scale; 0 = best, 100 = worst
c Scale 0 to 68
d Scale 0 to 66
Placebo/MTX Kineret/MTX
100 mg/day(n = 251) (n = 250) Parameter (median) Baseline Month 6 Baseline Month 6 Patient Reported Outcomes Disability indexa 1.38 1.13 1.38 1.00 Patient global assessmentb 51.0 41.0 51.0 29.0 Painb 56.0 44.0 63.0 34.0 Objective Measures ESR (mm/hr) 35.0 32.0 36.0 19.0 CRP (mg/dL) 2.2 1.6 2.2 0.5 Physician's Assessments Tender/painful jointsc 20.0 11.0 23.0 9.0 Physician global assessmentb 59.0 31.0 59.0 26.0 Swollen jointsd 18.0 10.5 17.0 9.0 A 24-week study was conducted in 242 patients with active RA on background methotrexate who were randomized to receive either etanercept alone or the combination of Kineret and etanercept. The ACR50 response rate was 31% for patients treated with the combination of Kineret and etanercept and 41% for patients treated with etanercept alone, indicating no added clinical benefit of the combination over etanercept alone. Serious infections were increased with the combination compared to etanercept alone [see Warnings and Precautions (5.1)].
In Study 1, the effect of Kineret on the progression of structural damage was assessed by measuring the change from baseline at month 12 in the Total Modified Sharp Score (TSS) and its subcomponents, erosion score, and joint space narrowing (JSN) score.2 Radiographs of hands/wrists and forefeet were obtained at baseline, 6 months and 12 months and scored by readers who were unaware of treatment group. A difference between placebo and Kineret for change in TSS, erosion score (ES) and JSN score was observed at 12 months (Table 5).
Table 5: Mean Radiographic Changes Over 12 Months in Study 1 * Differences and 95% confidence intervals for the differences in change scores between Placebo/MTX and Kineret/MTX
** Based on Wilcoxon rank-sum test
Placebo/MTX
(N = 450)Kineret 100 mg/day
/MTX
(N = 449)Placebo/MTX vs.
Kineret/MTXBaseline Change at Month 12 Baseline Change at Month 12 95% Confidence Interval* p-value** TSS 52 2.6 50 1.7 0.9 [0.3, 1.6] < 0.001 Erosion 28 1.6 25 1.1 0.5 [0.1, 1.0] 0.024 JSN 24 1.1 25 0.7 0.4 [0.1, 0.7] < 0.001 The disability index of the Health Assessment Questionnaire (HAQ) was administered monthly for the first six months and quarterly thereafter during Study 1. Health outcomes were assessed by the Short Form-36 (SF-36) questionnaire. The 1-year data on HAQ in Study 1 showed more improvement with Kineret than placebo. The physical component summary (PCS) score of the SF-36 also showed more improvement with Kineret than placebo but not the mental component summary (MCS).
14.2 Clinical Studies in NOMID
The efficacy of Kineret was evaluated in a prospective, long-term, open-label and uncontrolled study which incorporated a withdrawal period in a subset of 11 patients. This study included 43 NOMID patients 0.7 to 46 years of age treated for up to 60 months. Patients were given an initial Kineret dose of 1–2.4 mg/kg body weight. During the study, the dose was adjusted by 0.5 to 1 mg/kg increments to a protocol-specified maximum of 10 mg/kg daily, titrated to control signs and symptoms of disease. The maximum dose actually studied was 7.6 mg/kg/day. The average maintenance dose was 3 to 4 mg/kg daily. In general, the dose was given once daily, but for some patients, the dose was split into twice daily administrations for better control of disease activity.
NOMID symptoms were assessed with a disease-specific Diary Symptom Sum Score (DSSS), which included the prominent disease symptoms fever, rash, joint pain, vomiting, and headache. In addition, serum amyloid A (SAA), hsCRP, and ESR levels were monitored. Changes in clinical and laboratory parameters from baseline to Months 3 to 6 and from Month 3 (before withdrawal) to the end of the withdrawal period were assessed in the subset of patients who underwent withdrawal. The estimated changes from baseline in DSSS are summarized through Month 60 in Table 6. Results were consistent across all subgroups, including age, gender, presence of CIAS1 mutation, and disease phenotype. Improvements occurred in all individual disease symptoms comprising the DSSS (Table 7), as well as in the serum markers of inflammation. For the 11 patients who went through a withdrawal phase, disease symptoms and serum markers of inflammation worsened after withdrawal and promptly responded to reinstitution of Kineret therapy. Upon withdrawal of treatment, the median time until disease flare criteria were met was 5 days.
Table 6. Estimated change from baseline in DSSS in NOMID patients (N=29) *Mean (SD) baseline value was 4.5 (3.2)
Time point Estimated mean change
from baseline in DSSS*95% confidence interval Month 3-6 -3.5 -3.7 to -3.3 Month 12 -3.6 -3.9 to -3.3 Month 36 -3.5 -3.8 to -3.2 Month 60 -3.5 -3.8 to -3.1 Table 7. Individual diary key symptom scores by visit (ITT diary population) *mean (SD)
Visit (month) Number of patients Fever score* Rash score* Joint pain score* Vomiting score* Headache score* Baseline 29 0.5 (0.8) 1.9 (1.1) 1.2 (1.1) 0.1 (0.2) 0.9 (1.0) 1 28 0.1 (0.1) 0.3 (0.5) 0.2 (0.3) 0.0 (0.0) 0.2 (0.3) 3 26 0.1 (0.2) 0.1 (0.2) 0.2 (0.4) 0.0 (0.1) 0.1 (0.2) 6 25 0.0 (0.1) 0.1 (0.1) 0.2 (0.4) 0.0 (0.1) 0.2 (0.3) 12 24 0.1 (0.1) 0.1 (0.2) 0.1 (0.2) 0.0 (0.1) 0.1 (0.2) 36 19 0.0 (0.2) 0.0 (0.2) 0.1 (0.3) 0.0 (0.0) 0.2 (0.6) 60 15 0.0 (0.0) 0.1 (0.3) 0.3 (0.7) 0.0 (0.0) 0.1 (0.3) Kineret treatment also appeared to be associated with improvement of, or stability in, assessments of other NOMID disease manifestations, such as CNS, audiogram, and visual acuity data, up to Month 60.
-
15 REFERENCES
- Cockcroft DW and Gault HM. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16:31-41.
- Sharp JT, Young DY, Bluhm GB, et al. How many joints in the hands and wrists should be included in a score of radiologic abnormalities used to assess rheumatoid arthritis? Arthritis Rheum. 1985; 28:1326-1335.
- National Cancer Institute. Surveillance, Epidemiology, and End Results Database (SEER) Program. SEER Incidence Crude Rates, 11 Registries, 1992-1999.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Kineret is supplied in single-use preservative free, prefilled glass syringes with 29 gauge needles. Each prefilled glass syringe contains 100 mg of anakinra per 0.67 mL. The full syringe contains 100 mg anakinra. Kineret is dispensed in a 4 x 7 syringe dispensing pack containing 28 syringes (NDC: 66658-234-28). Kineret is also dispensed in a 1 x 7 syringe dispensing pack containing 7 syringes (NDC: 66658-234-07).
-
17 PATIENT COUNSELING INFORMATION
Instruct patients and their caregivers on the proper dosage and administration of Kineret and provide all patients with the “Patient information and Instructions for Use” insert. While this Patient Information and Instructions for Use provides information about the product and its use, it is not intended to take the place of regular discussions between the patient and healthcare provider. The ability to inject subcutaneously should be assessed to ensure proper administration of Kineret. Thoroughly instruct patients and their caregivers on the importance of proper disposal and caution against the reuse of needles, syringes, and drug product. A puncture-resistant container for the disposal of used syringes should be available to the patient. The full container should be disposed of according to the directions provided by the healthcare provider.
Infections: Inform patients that Kineret may lower the ability of their immune system to fight infections. Advise patients of the importance of contacting their doctor if they develop any symptoms of infection.
Injection-site reactions: Physicians should explain to patients that almost a quarter of patients in the clinical trial experienced a reaction at the injection site. Injection-site reactions may include pain, erythema, swelling, purities, brusing, mass, inflammation, dermatitis, edema, urticaria, vesicles, warmth, and hemorrhage. Inform patients or their caregivers that the prefilled syringe should be removed from refrigeration and left at room temperature for 30 minutes before injecting. Patients should be cautioned to avoid injecting into an area that is already swollen or red. Any persistent reaction should be brought to the attention of the prescribing physician.
Allergic or other drug reactions: Inform patients about the signs and symptoms of allergic and other adverse drug reactions and the appropriate actions they should take if they experience any of these signs and symptoms.
See FDA-approved patient labeling (Patient Information and Instruction for Use)
sobi
SWEDISH ORPHAN BIOVITRUMManufactured by:
Swedish Orphan Biovitrum AB (publ)
SE-112 76 Stockholm, SwedenU.S. License No. 1859
© Swedish Orphan Biovitrum AB (publ). All rights reserved.
The product, its production and/or its use may be covered by one or more US Patents, including US Patent Nos. 6,599,873 and 6,858,409 as well as other patents or patents pending.
-
PATIENT PACKAGE INSERT
Patient Information
Kineret®(KIN-eh-ret)
(anakinra)
injection, for subcutaneous use
Read this Patient Information before you start using Kineret and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What is Kineret?
Kineret is a prescription medicine called an interleukin-1 receptor antagonist (IL-1ra) used to:
- Reduce the signs and symptoms and slow the damage of moderate to severe active rheumatoid arthritis (RA) in people age 18 years and older when 1 or more other drugs for RA have not worked.
- Treat people with a form of Cryopyrin-Associated Periodic Syndromes (CAPS) called Neonatal-Onset Multisystem Inflammatory Disease (NOMID)
Kineret is not for children with Juvenile Rheumatoid Arthritis
Who should not use Kineret?
Do not use Kineret if you are allergic to:
- proteins made from bacteria called E.Coli. Ask your healthcare provider if you are not sure.
- anakinra or any of the ingredients in Kineret. See the end of this leaflet for a complete list of ingredients in Kineret.
What should I tell my healthcare provider before using Kineret?
Before you use Kineret, tell your healthcare provider if you:
- have an infection, a history of infections that keep coming back or other problems that can increase your risk of infections.
- have kidney problems.
- are scheduled to receive any vaccines. People using Kineret should not receive live vaccines.
- are pregnant or plan to become pregnant. It is not known if Kineret will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Kineret passes into your breast milk. You and your healthcare provider should decide if you will use Kineret or breastfeed.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Kineret and other medicines may affect each other and cause serious side effects.
Especially, tell your healthcare provider if you take certain other medicines that:
- affect your immune system called Tumor Necrosis Factor (TNF) blockers
Ask your healthcare provider for a list of these medicines if you are not sure.
Know the medicines you take. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new prescription.
How should I use Kineret?
- Read the Instructions for Use at the end of this Patient Information for information about the right way to use Kineret.
- Use Kineret exactly as your healthcare provider tells you to.
- You may not have to use all of the liquid medicine in the prefilled syringe. Your healthcare provider will show you how to find the correct dose of Kineret for you or your child.
- Kineret is given by injection under your skin.
- Inject Kineret at about the same time each day.
- If you have a kidney problem your healthcare provider may need to change how often you use your Kineret injections.
- If you miss a dose of Kineret, talk to your healthcare provider to find out when you should use your next injection.
What are the possible side effects of Kineret?
Kineret may cause serious side effects, including:
-
serious infections. Kineret may lower your ability to fight infections. During your treatment with Kineret, call your healthcare provider right away if you:
- get an infection
- have any sign of an infection including a fever or chills
- have any open sores on your body
You may get an infection if you receive live vaccines while you use Kineret. You should not receive live vaccines while you use Kineret.
-
allergic reactions. Stop using Kineret and call your healthcare provider or get emergency help right away if you have any of these symptoms of an allergic reaction:
- swelling of your face, lips, mouth or tongue
- trouble breathing
- wheezing
- severe itching
- skin rash, redness, or swelling outside of the injection site area
- dizziness or fainting
- fast heartbeat or pounding in your chest (tachycardia)
- sweating
- decreased ability of your body to fight infections (immunosuppression). It is not known if treatment with medicines that cause immunosuppression, like Kineret, affect your risk of getting cancer.
- low white blood cell count (neutropenia). Kineret may cause you to have a lower number of certain white blood cells (neutrophils). Neutrophils are important in fighting infections. You should have blood tests before starting treatment with Kineret, then monthly for 3 months. After the first 3 months you should have your blood tested every 3 months for up to 1 year.
The most common side effects of Kineret include:
- injection site skin reactions. The symptoms of injection site skin reactions may include:
- redness
- swelling
- stinging
- bruising
- itching
Most injection site reactions are mild, happen early during treatment, and last about 14 to 28 days. Injection site reactions have been observed less frequently in people with NOMID.
- rheumatoid arthritis (RA) gets worse even with treatment, if you already have RA
- headache
- nausea and vomiting
- diarrhea
- joint pain
- fever
- feeling like you have the flu
- sore throat or runny nose
- sinus infection
- pain in your stomach area
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of Kineret. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Kineret?
- Store Kineret in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Do not freeze or shake Kineret.
- Keep Kineret in its original carton and away from light.
Keep Kineret and all medicines out of the reach of children.
General Information about the safe and effective use of Kineret.
Medicines are sometimes prescribed for purposes other than those listed in a patient leaflet. Do not use Kineret for a condition for which it was not prescribed. Do not give Kineret to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information leaflet summarizes the most important information about Kineret. If you would like more information about Kineret, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about Kineret that is written for health professionals.
For more information go to www.kineretrx.com or call 1-866-547-0644.
What are the ingredients in Kineret?
Active ingredients: anakinra
Inactive ingredients: anhydrous citric acid, disodium EDTA, polysorbate 80, and sodium chloride in Water for Injection, USP
-
INSTRUCTIONS FOR USE
Instructions for Use
Kineret®(KIN-eh-ret)
(anakinra)
injection, for subcutaneous use
Read this Instructions for Use before you start using Kineret and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
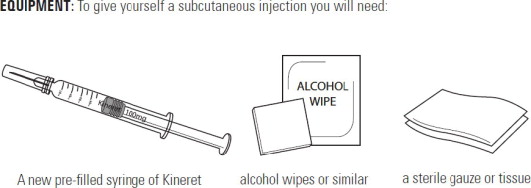
Supplies you will need to give your Kineret injection: See Figure A.
- 1 Kineret prefilled syringe
- 1 alcohol wipe
- 1 dry sterile gauze or tissue
- 1 puncture-resistant sharps disposal container
Each Kineret dose comes in a prefilled glass syringe. There are 7 syringes in each new Kineret box, 1 for each day of the week. Use a new Kineret syringe each day. Use the Kineret prefilled syringe that matches the day of the week until all 7 syringes are used.
Setting up for your injection:
Step 1. Take the carton containing the prefilled syringes of Kineret out of the refrigerator. Remove the prefilled syringe from the box that matches the day of the week. Put the carton containing the remaining prefilled syringes back in the refrigerator.
Step 2. Find a clean, flat work surface, such as a table.
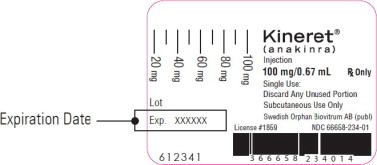
Step 3. Check the expiration date on the syringe label. See Figure B.
If the expiration date has passed, do not use the syringe. Call your pharmacist or call 1-866-773-5274 for assistance.
Step 4. Take the Kineret prefilled syringe out of the refrigerator and leave it in room temperature for 30 minutes before your injection.
Make sure the liquid medicine in the prefilled syringe is clear and colorless. It is normal to see a small amount of tiny particles that are white, or that you can see through. Do not inject the medicine if it is cloudy or discolored, or has large or colored particles. Call your healthcare provider or pharmacist if you have any questions about your Kineret prefilled syringe.
Step 5. Gather all the supplies you will need for your injection.
Step 6. Wash your hands with soap and warm water.
Preparing your correct dose of Kineret:
-
Preparing a 100 mg dose of Kineret:
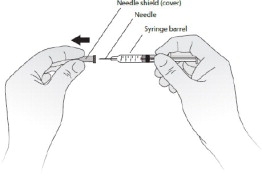
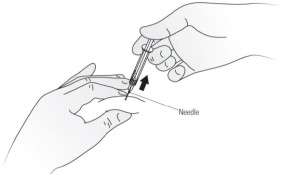
- Hold the syringe barrel and pull the cover straight off the needle. See Figure C. Do not touch the needle or push the plunger. Throw away the needle cover.
- You may notice a small air bubble in the prefilled syringe. You do not have to remove the air bubble before injecting. Injecting the solution with the air bubble is harmless.
- Carefully place the barrel of the syringe on the table until you are ready to inject. Do not let the needle touch the table. Do not recap the needle.
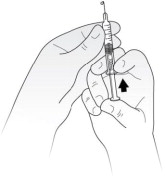
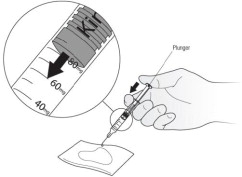
How to prepare a dose of Kineret less than 100 mg:
- Hold the syringe in 1 hand with the needle pointing straight upwards. See Figure D. Put your thumb on the plunger rod and push slowly until you see a tiny liquid drop at the tip of the needle.
- Turn the syringe so that the needle is now pointing downwards. Place a sterile gauze or tissue on a flat surface and hold the syringe above it with the needle pointing towards the gauze or tissue. See Figure E. Make sure the needle does not touch the gauze or tissue.
- Put your thumb on the plunger rod and push slowly until you can see that the top of the plunger has reached the correct number for your Kineret dose your healthcare provider has prescribed. The liquid that was pushed out of the needle will be absorbed by the gauze or tissue. See Figure E.
- If you are not able to select the correct dose of Kineret, throw away the syringe and use a new one.
- Carefully place the barrel of the syringe on the table until you are ready to inject. Do not let the needle touch the table. Do not recap the needle.
Selecting and preparing the injection site:
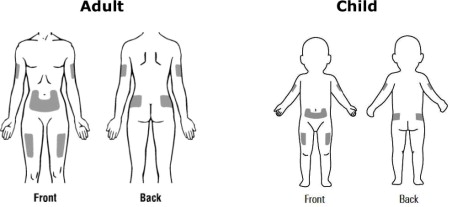
Step 7. Choose an injection site. See Figure F.
Recommended injection sites for adults and children include:
- outer area of the upper arms
- abdomen (except the 2-inch area around the belly button)
- front of the middle thighs
- upper outer areas of the buttocks
Choose a new site each time you use Kineret. Choosing a new site may help avoid soreness at 1 site. Do not inject Kineret into an area of skin that is tender, red, bruised, swollen, or hard. Avoid areas of skin with scars or stretch marks. Do not inject Kineret close to a vein that you can see under the surface of your skin.
- Clean your injection site with an alcohol swab. Let the area dry completely.
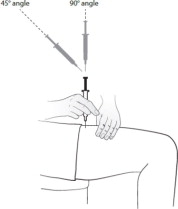
Giving your injection:
Step 8. Gently pinch a fold of skin at the cleaned injection site.Step 9. With your other hand, hold the syringe like a pencil at a 45 degree to 90 degree angle to the skin. With a quick, dart-like motion insert the needle into the skin. See Figure G.
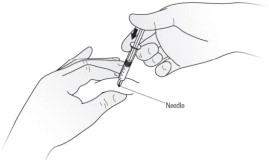
Step 10. After the needle is inserted into the skin, slowly push the plunger all the way down to inject Kineret. See Figure H.
Step 11. When the syringe is empty, pull the needle out of the skin while carefully keeping the needle at the same angle as inserted. See Figure I.
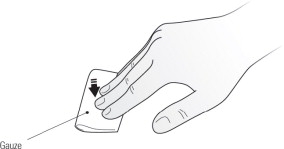
Step 12. Place a dry cotton ball or gauze pad over the injection site and press for several seconds. See Figure J. Do not use an alcohol swab as it may cause stinging. If there is a little bleeding, you may cover the injection site with a small bandage.
Important Information about your Kineret prefilled syringe:
- Use each Kineret prefilled syringe only 1 time. Do not use a syringe more than 1 time. Do not recap a needle.
- You may not have to use all of the liquid medicine in the prefilled syringe. Your healthcare provider will show you how to find the correct dose of Kineret for you or your child.
- If you notice that some medicine is left in the prefilled syringe, do not inject again with the same prefilled syringe.
- If you drop a prefilled syringe, do not use it. The glass syringe may be broken, or the needle may be bent or dirty. Throw away the prefilled syringe and replace it with a new one. Take a new prefilled syringe from what would be the last day of the week in your current box. For example, if you start on Wednesday, the last day of the week in your series is Tuesday. After using all the remaining prefilled syringes in your current box, start your next box of Kineret prefilled syringes.
Disposal of your Kineret syringes:
- Put your used syringes in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose syringes in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- upright and stable during use
- leak-resistant
- properly labeled to warn of hazardous waste inside the container
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- Throw away the wet gauze or tissue with your syringe and clean the table surface with a fresh swab.
This Patient Information and Instructions for Use has been approved by the U.S. Food and Drug Administration.
sobi
SWEDISH ORPHAN BIOVITRUMManufactured by:
Swedish Orphan Biovitrum AB (publ)
SE-112 76 Stockholm, Sweden
License No. 1859© Swedish Orphan Biovitrum AB (publ). All rights reserved.
Revised Date: 09/2015
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel - Carton Label
4 x 7 Syringe Dispensing Packs (28 x 100 mg/0.67 mL Prefilled Glass Syringes) NDC: 66658-234-28
sobi
Kineret®
(anakinra)Injecton
100 mg/0.67 mL
Single Use: Discard Any Unused Portion
Subcutaneous Use Only
Graduated Prefilled Glass Syringes with 29 Gauge Needles
Sterile Solution - No Preservative
Manufactured by Swedish Orphan Biovitrum AB (publ)
SE-112 76 Stockholm, Sweden
License # 1859 Rx Only
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel - Carton Label
(Seven x 100 mg/0.67 mL Prefilled Glass Syringes) NDC: 66658-234-07
sobi
Kineret®
(anakinra)Injecton
100 mg/0.67 mL
Single Use: Discard Any Unused Portion
Subcutaneous Use Only
Graduated Prefilled Glass Syringes with 29 Gauge Needles
Sterile Solution - No Preservative
Refrigerate at 2° to 8°C (36° to 46°F). Do Not Freeze or Shake.
Protect from Light.
Rx Only
Manufactured by Swedish Orphan Biovitrum AB (publ)
SE-112 76 Stockholm, Sweden
License # 1859
-
INGREDIENTS AND APPEARANCE
KINERET
anakinra injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66658-234 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength anakinra (UNII: 9013DUQ28K) (anakinra - UNII:9013DUQ28K) anakinra 100 mg in 0.67 mL Inactive Ingredients Ingredient Name Strength anhydrous citric acid (UNII: XF417D3PSL) 1.29 mg in 0.67 mL sodium chloride (UNII: 451W47IQ8X) 5.48 mg in 0.67 mL edetate disodium (UNII: 7FLD91C86K) 0.12 mg in 0.67 mL polysorbate 80 (UNII: 6OZP39ZG8H) 0.70 mg in 0.67 mL Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66658-234-07 7 in 1 CARTON 12/15/2009 1 0.67 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC: 66658-234-28 4 in 1 CASE 12/15/2009 2 NDC: 66658-234-07 7 in 1 CARTON 2 0.67 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103950 12/15/2009 Labeler - Swedish Orphan Biovitrum AB (publ) (354010589) Establishment Name Address ID/FEI Business Operations Swedish Orphan Biovitrum AB (publ), Sweden 776024627 MANUFACTURE(66658-234)
Trademark Results [Kineret]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 KINERET 98059621 not registered Live/Pending |
KENOVER MARKETING CORP. 2023-06-26 |
 KINERET 77837366 3875628 Live/Registered |
Royal Wine Corporation 2009-09-29 |
 KINERET 75538963 2585682 Live/Registered |
SWEDISH ORPHAN BIOVITRUM AB (PUBL) 1998-08-19 |
 KINERET 75318977 not registered Dead/Abandoned |
Amgen Inc. 1997-07-03 |
 KINERET 73778156 1588062 Dead/Cancelled |
KINERET FOODS CORPORATION 1989-02-02 |
 KINERET 73655240 1488691 Dead/Cancelled |
KINERET FOODS CORPORATION 1987-04-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.