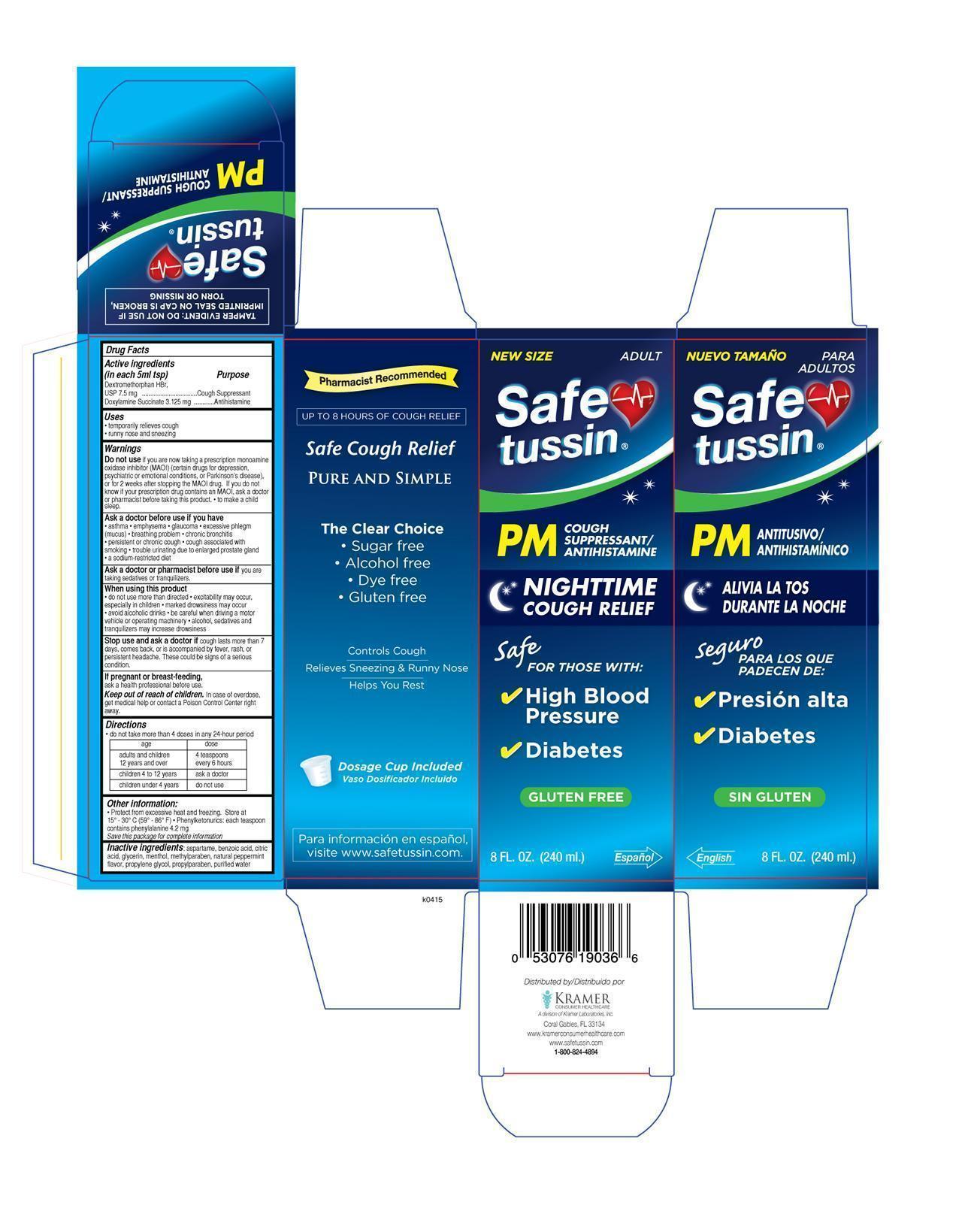
SAFETUSSIN PM- dextromethorphan doxylamine succinate liquid
Safetussin by
Drug Labeling and Warnings
Safetussin by is a Otc medication manufactured, distributed, or labeled by Kramer Laboratories, Denison Pharmecuticals. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
-
WARNINGS
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product. to make a child sleep. - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SAFETUSSIN PM
dextromethorphan doxylamine succinate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 55505-190 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 7.5 mg in 5 mL DOXYLAMINE SUCCINATE (UNII: V9BI9B5YI2) (DOXYLAMINE - UNII:95QB77JKPL) DOXYLAMINE SUCCINATE 3.125 mg in 5 mL Inactive Ingredients Ingredient Name Strength Aspartame (UNII: Z0H242BBR1) benzoic acid (UNII: 8SKN0B0MIM) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) glycerin (UNII: PDC6A3C0OX) menthol (UNII: L7T10EIP3A) methylparaben (UNII: A2I8C7HI9T) MINT (UNII: FV98Z8GITP) propylene glycol (UNII: 6DC9Q167V3) propylparaben (UNII: Z8IX2SC1OH) water (UNII: 059QF0KO0R) Product Characteristics Color Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55505-190-36 240 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 07/01/2015 Labeler - Kramer Laboratories (122720675) Establishment Name Address ID/FEI Business Operations Denison Pharmecuticals 001207208 manufacture(55505-190)
Trademark Results [Safetussin]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SAFETUSSIN 78592800 3068103 Live/Registered |
Kramer Laboratories, Inc. 2005-03-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.