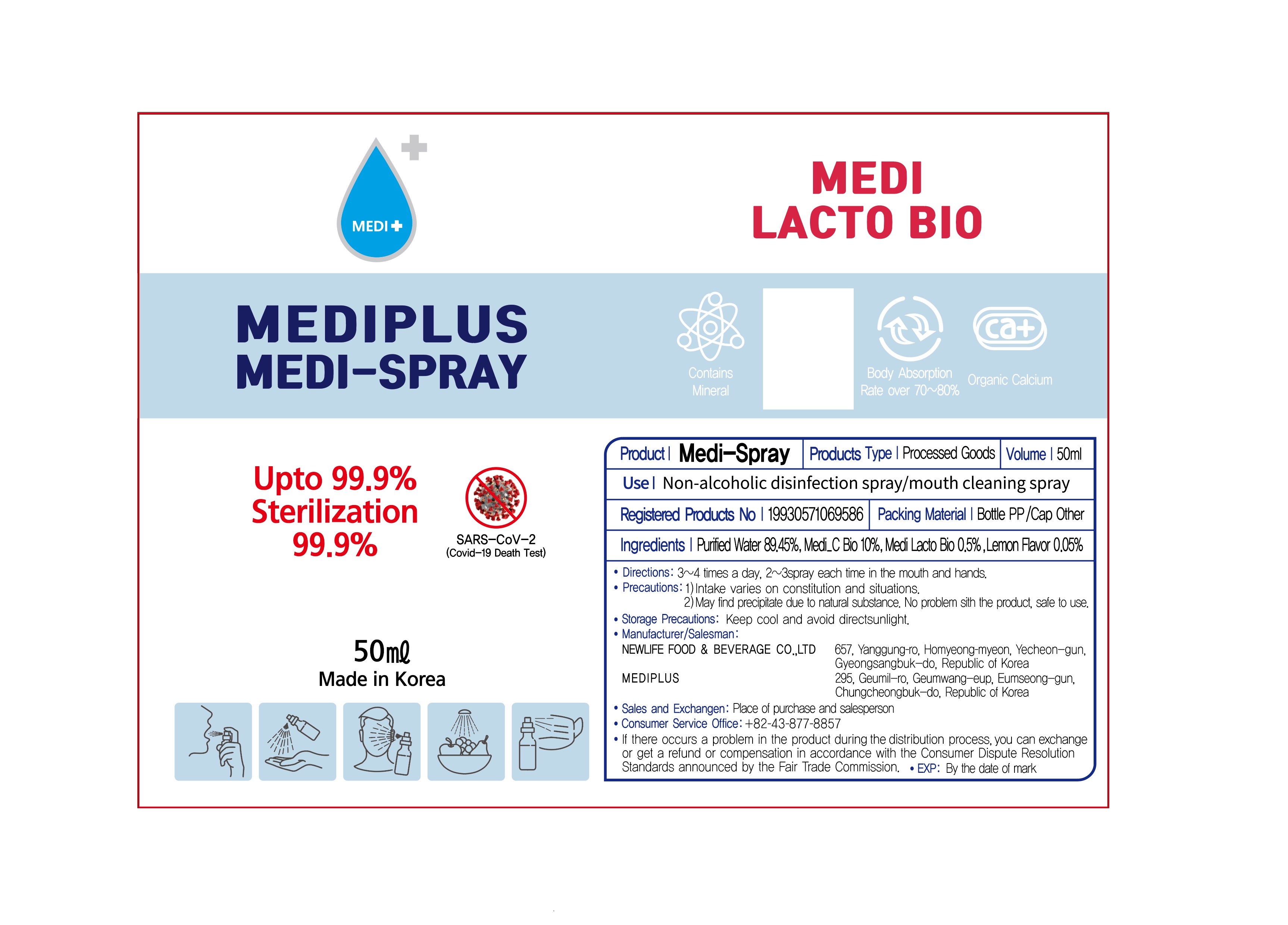
MEDISPRAY- calcined weathered sedimentary coral powder liquid
MEDISPRAY by
Drug Labeling and Warnings
MEDISPRAY by is a Otc medication manufactured, distributed, or labeled by MEDIPLUS, K.Boeun Pharmaceutical Co.,Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
Do not use
- in children less than 2 months of age
Do not spray directly with your eyes open. If it gets into your eyes and causes irritation, rinse thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition.
Keep out of reach of children.
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MEDISPRAY
calcined weathered sedimentary coral powder liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 82622-0001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 10 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) LEMON (UNII: 24RS0A988O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 82622-0001-1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/09/2020 Labeler - MEDIPLUS (695439402) Registrant - MEDIPLUS (695439402) Establishment Name Address ID/FEI Business Operations K.Boeun Pharmaceutical Co.,Ltd. 695674074 manufacture(82622-0001)
Trademark Results [MEDISPRAY]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MEDISPRAY 75482435 2255589 Dead/Cancelled |
Bartolomei, Alberto S. 1998-05-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.