BioShell Oral Antiseptic Spray
BioShell Oral Antiseptic by
Drug Labeling and Warnings
BioShell Oral Antiseptic by is a Otc medication manufactured, distributed, or labeled by BioFilm Inc, TRI-PAC, INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
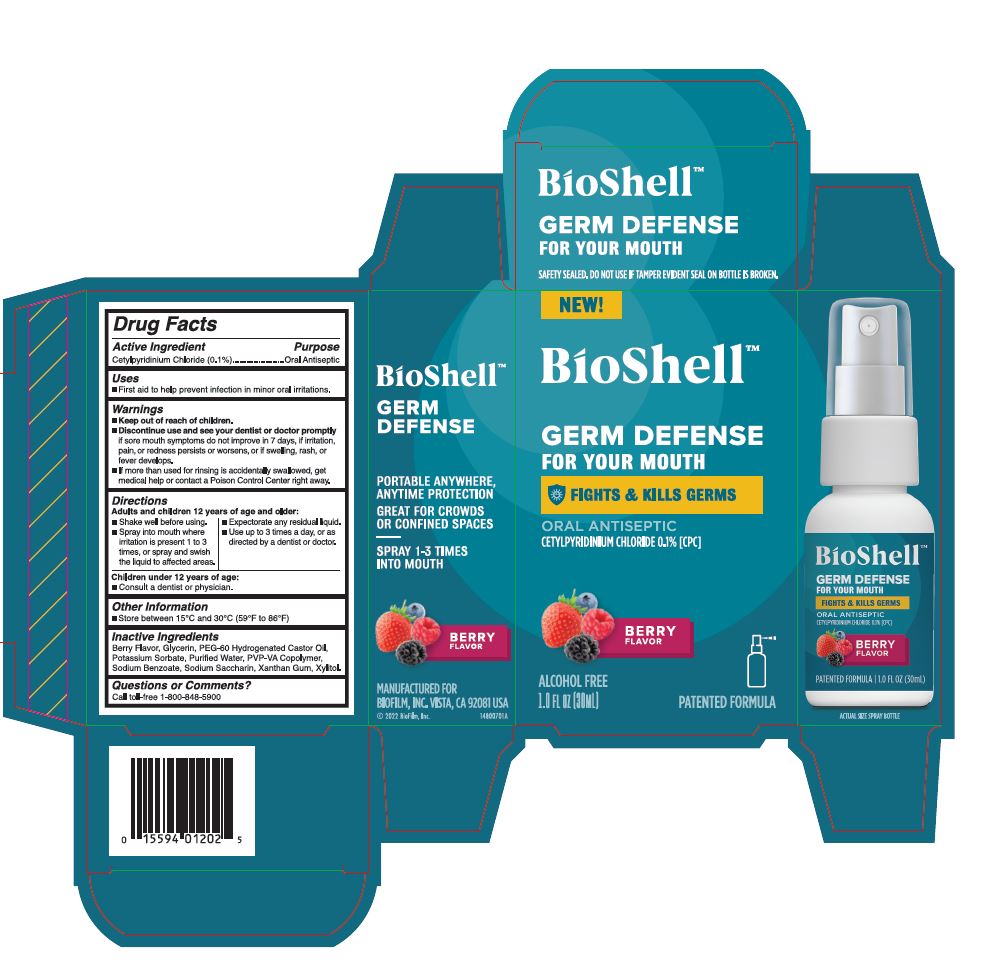
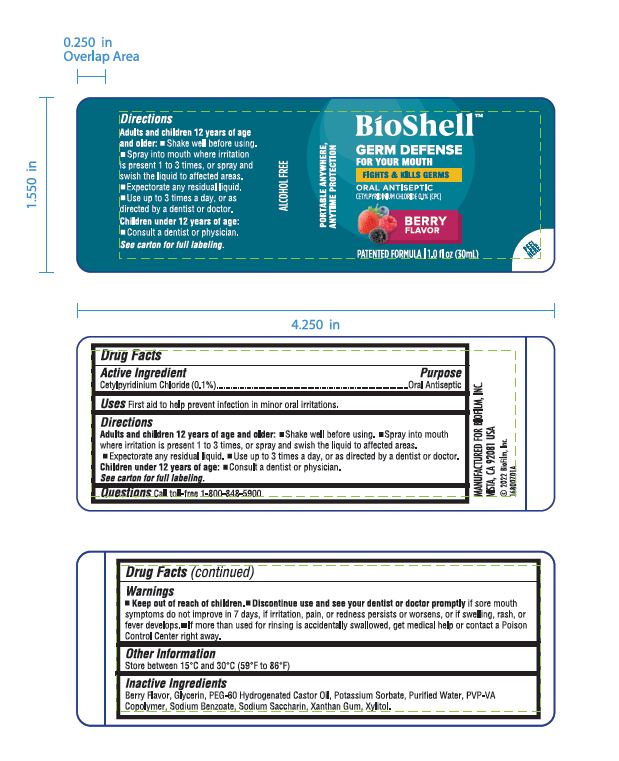
BIOSHELL ORAL ANTISEPTIC- cetylpyridinium chloride liquid
BioFilm Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
BioShell Oral Antiseptic Spray
Warnings
Discontinue use and see your dentist or doctor promplty if sore mouth symptoms do not improve in 7 days, if irritation, pain, or redness persists or worsens, or if swelling, rash, or fever develops.
If more than used for rinsing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Adults and children 12 years of age and older
- Shake well before using
- Spray into mouth where irritation is present 1 to 3 times, or spray and swish the liquid to affected areas
- Expectorate any residual liquid
- Use up to 3 times a day, or as directed by a dentist or doctor.
- Children under 12 years of age
- Consult a dentist or physician
| BIOSHELL ORAL ANTISEPTIC
cetylpyridinium chloride liquid |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - BioFilm Inc (790780258) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| TRI-PAC, INC. | 020844956 | manufacture(66357-030) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.