FILSUVEZ- birch triterpenes gel
FILSUVEZ by
Drug Labeling and Warnings
FILSUVEZ by is a Prescription medication manufactured, distributed, or labeled by Amryt Pharmaceuticals DAC, Eurofins BioPharma Product Testing Hamburg GmbH, Amryt GmbH, CIP Chemisches Institut Pforzheim GmbH, BioChem Labor für biologische und chemische Analytik GmbH, Nuvisan GmbH, Lichtenheldt GmbH, Synergy Health Radeberg GmbH, Pharma Packaging Solutions (Carton Service, Inc.). Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use FILSUVEZ safely and effectively. See full prescribing information for FILSUVEZ.
FILSUVEZ® (birch triterpenes) topical gel
Initial U.S. Approval: 2023INDICATIONS AND USAGE
FILSUVEZ topical gel is indicated for the treatment of wounds associated with dystrophic and junctional epidermolysis bullosa in adult and pediatric patients 6 months of age and older. (1)
DOSAGE AND ADMINISTRATION
- Apply a 1 mm layer of FILSUVEZ to the affected wound surface and cover with wound dressing or apply FILSUVEZ directly to dressing so that the topical gel is in direct contact with the wound. Do not rub in the topical gel. (2)
- Apply FILSUVEZ at wound dressing changes until the wound is healed. (2)
- Each tube of FILSUVEZ is for one-time use only. (2)
- For topical use; not for oral, intravaginal, intra-anal, or ophthalmic use. (2)
DOSAGE FORMS AND STRENGTHS
Topical gel: 10% birch triterpenes w/w supplied in 25 mL sterile tubes (3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
- Hypersensitivity Reactions: If signs or symptoms of hypersensitivity occur, discontinue use immediately and initiate appropriate therapy. (5.1)
ADVERSE REACTIONS
The most common (incidence ≥2%) adverse reactions are application site reactions. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Amryt Pharmaceuticals DAC at 1-855-303-2347 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
- Wash hands before and after applying FILSUVEZ or wear gloves for application.
- Apply a 1 mm layer of FILSUVEZ to the affected wound surface only. Do not rub in the gel. Cover the wound with a sterile non-adhesive wound dressing. Alternatively, apply FILSUVEZ directly to the dressing so that the topical gel is in direct contact with the wound.
- Apply FILSUVEZ to cleansed wounds with wound dressing changes until the wound is healed.
- If a FILSUVEZ-treated wound becomes infected, discontinue treatment to that wound until the infection has resolved.
- Each tube of FILSUVEZ is for one-time use only. Once the tube is opened, use the product immediately. Discard the tube after use in household trash or through a drug take back site, if available.
- Avoid contact of FILSUVEZ with eyes and mucous membranes (e.g., mouth, vagina, anus). In case of accidental contact, irrigate the area with water.
- FILSUVEZ is for topical use only. Not for use on mucous membranes (oral, intravaginal, or intra-anal). Not for ophthalmic use.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed elsewhere in the labeling:
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of FILSUVEZ was evaluated in EASE, a randomized, double-blind, multicenter, placebo-controlled trial in 223 adult and pediatric subjects with inherited EB. During the double-blind phase of EASE, subjects received topical treatment with either FILSUVEZ or a placebo gel on partial-thickness wounds every 1 to 4 days for a total of 90 days. Treated wounds were covered with non-adhesive dressings. Following completion of the double-blind phase, all subjects received FILSUVEZ for a total of 24 months during the open-label phase [see Clinical Studies (14)].
Table 1 presents adverse reactions that occurred in at least 2% of subjects treated with FILSUVEZ during the 90-day double-blind phase of EASE and at a greater frequency than in the placebo gel group.
Table 1: Number (%) of Subjects with Adverse Reactions Occurring in ≥ 2% Adverse Reaction FILSUVEZ
(N=109)
n (%)Placebo Gel
(N=114)
n (%)- * Includes: application site pruritus, administration site pain, administration site pruritus.
Application site reaction* 8 (7.3) 7 (6.1) Squamous cell carcinoma of the skin (SCC) was reported as an adverse event in the double-blind and open-label periods of EASE. Four subjects with recessive dystrophic EB each reported one SCC: a 20-year-old male on day 1 of the double-blind period; three female subjects ages 22, 46, and 49 years during the open-label period. Two of the four subjects had applied FILSUVEZ to the area which developed the SCC.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data with use of FILSUVEZ in pregnant women to evaluate for drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. In an animal reproduction study, oral administration of birch triterpenes to pregnant rats during the period of organogenesis had no effects on reproductive or fetal parameters (see Data).
Systemic absorption of FILSUVEZ in humans is low following topical administration of FILSUVEZ, and maternal use is not expected to result in fetal exposure to the drug [see Pharmacokinetics (12.3)].
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In an embryofetal development study, birch triterpenes were orally administered to pregnant rats at doses of 10, 30, or 100 mg/kg/day during the period of organogenesis. Birch triterpenes did not cause maternal toxicity or fetal malformations at doses up to 100 mg/kg/day. In a prenatal and postnatal development study, birch triterpenes were orally administered to pregnant rats at doses of 10, 30, or 100 mg/kg/day from gestation day 5 through lactation day 20. Birch triterpenes did not affect development at doses up to 100 mg/kg/day. The available data do not support relevant comparisons of systemic birch triterpenes exposures achieved in the animal studies to exposures observed in humans after topical use of FILSUVEZ.
8.2 Lactation
Risk Summary
There are no data on the presence of birch triterpenes or metabolites in human milk, the effects on the breastfed infant, or the effect on milk production.
No effects on the breastfed infant are anticipated since the systemic exposure of the breastfeeding woman to FILSUVEZ would be low. Therefore, the developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for FILSUVEZ and any potential adverse effects on the breastfed infant from FILSUVEZ or from the underlying maternal condition [see Pharmacokinetics (12.3)].
8.4 Pediatric Use
The safety and effectiveness of FILSUVEZ for the treatment of wounds associated with dystrophic and junctional EB have been established in pediatric patients 6 months of age and older. Use of FILSUVEZ in this age group is supported by evidence from a single randomized, placebo-controlled trial in 156 subjects 6 months to 17 years of age [see Clinical Studies (14)].
The safety and effectiveness of FILSUVEZ have not been established in pediatric patients younger than 6 months of age.
-
11 DESCRIPTION
FILSUVEZ (birch triterpenes) topical gel is a sterile botanical drug product for topical use and contains birch triterpenes in an oil base. FILSUVEZ is a colorless to slightly yellowish, opalescent, non-aqueous gel.
Birch triterpenes is a botanical drug substance composed of a mixture of pentacyclic triterpenes. The botanical drug substance is a dry extract, refined, from birch bark from Betula pendula Roth, Betula pubescens Ehrh., as well as hybrids of both species, quantified to 72-88% (w/w) betulin, 2.4-5.7% (w/w) lupeol, 2.6-4.2% (w/w) betulinic acid, 0.5-1.2% (w/w) erythrodiol, 0.3-0.8% (w/w) oleanolic acid.
The structural formulae of the main triterpene constituents are shown in Figure 1.
Figure 1: Structure of Triterpene Constituents
Betulin 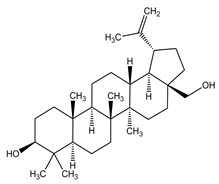
Erythrodiol 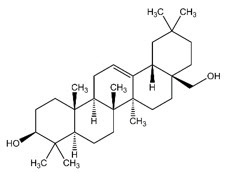
Betulinic acid 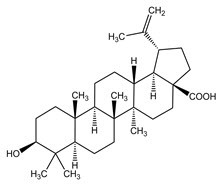
Oleanolic acid 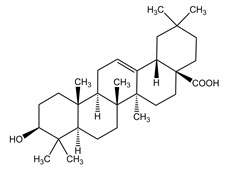
Lupeol 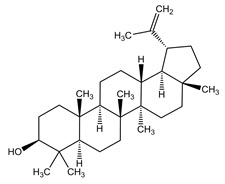
Each gram of FILSUVEZ topical gel 10% (w/w) contains 100 mg of birch triterpenes in an oil base of refined sunflower oil. FILSUVEZ contains no additional excipients.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of FILSUVEZ in the treatment of wounds associated with epidermolysis bullosa is unknown.
12.3 Pharmacokinetics
Absorption
Systemic exposure to betulin was assessed in the 66 evaluable subjects aged ≥13 months to ≤52 years with dystrophic and junctional EB in the clinical trial EASE using venous blood sampling and validated liquid chromatography with tandem mass spectrometry assay.
Following treatment with FILSUVEZ once daily (n=27), every 2 (n=33) or 3 days (n=4) or once weekly (n=2) for 90 days with a mean treatment area of 12% body surface area or affected wound surface area of 0.11 m2 at baseline, betulin blood concentrations in 68% subjects (n=45) were below the lower limit of quantification of 10 ng/mL on Day 90. The highest concentrations of betulin in adult and pediatric subjects with a median age of 10 years (range: ≥13 months to <18 years) were 33 ng/mL and 207 ng/mL, respectively, which were observed on Day 90.
Metabolism
The in vitro study indicated that incubation of 1 µmol/L betulin with human hepatocytes generated several unidentified phase I and/or phase II metabolites. In vitro, betulin was mainly metabolized by cytochrome P450 (CYP)3A. The relative contribution of CYP3A and phase II metabolizing enzymes in the overall metabolism of betulin has not been fully characterized.
Drug Interaction Studies
Clinical Studies
No clinical studies evaluating the drug interaction potential of FILSUVEZ have been conducted.
In Vitro Studies
CYP Enzymes: Betulin inhibited CYP3A and CYP2C8 with an IC50 value of >0.17 µmol/L (75 ng/mL). Betulin up to 10 µmol/L (4427 ng/mL) did not induce CYP 1A2, 2B6, or 3A4. These findings suggest that FILSUVEZ has no clinically meaningful effect on the PK of drugs metabolized by CYP 1A2, 2B6, 2C8, 2C9, 2C19, 2D6, or 3A.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity studies have been performed with FILSUVEZ or birch triterpenes.
Birch triterpenes were not genotoxic in the in vitro bacterial mutagenicity (Ames) assay, an in vitro mammalian chromosome aberration assay using human peripheral lymphocytes, or at doses up to 500 mg/kg in an in vivo mouse micronucleus assay.
In a fertility and early embryonic development study in rats, birch triterpenes were orally administered at doses of 10, 30, or 100 mg/kg/day from 2 weeks before mating through gestation day 6. Birch triterpenes had no effects on mating or fertility in male or female rats at doses up to 100 mg/kg/day.
-
14 CLINICAL STUDIES
The efficacy of FILSUVEZ for the treatment of partial-thickness wounds associated with inherited EB was evaluated in a randomized, double-blind, placebo-controlled trial in adults and pediatric subjects 6 months of age and older (EASE; NCT03068780) with dystrophic EB (DEB) and junctional EB (JEB). Subjects were randomized 1:1 to receive FILSUVEZ (n=109) or placebo topical gel (n=114) and instructed to apply approximately 1 mm (0.04 inch) of the investigational product to all their wounds at each dressing change (every 1 to 4 days) for 90 days. At randomization, 1 wound was selected by the investigator as the target wound for the evaluation of the primary efficacy endpoint. The target wound was defined as a partial-thickness wound of 10-50 cm2 in surface area and present for 21 days to 9 months prior to screening.
Of the 223 subjects randomized, the median age was 12 years (range: 6 months to 81 years), 70% were under 18 years of age, and 60% were male and 40% were female. Eighty three (83)% of subjects were White, 5% were Asian, 1% were Black or African American, and 10% were other races or did not have race recorded. For ethnicity, 35% identified as Hispanic or Latino and 65% identified as not Hispanic or Latino. Of these 223 subjects, 195 had DEB, of which 175 subjects had recessive DEB (RDEB) and 20 had dominant DEB (DDEB); in addition, there were 26 subjects with JEB and 2 subjects with EB simplex.
The primary endpoint was the proportion of subjects with first complete closure of the target wound by Day 45 of the 90-day double-blind phase of the study, based on clinical assessment by the investigator. Efficacy results from EASE are presented in Table 2.
Table 2: Efficacy Results for the Treatment of Partial-Thickness Wounds in Subjects with EB in Trial EASE (Full Analysis Set) Efficacy Parameter FILSUVEZ
N=109Placebo Gel
N=11495% CI for the Treatment Difference CI=Confidence interval - * Two subjects with EB simplex are not included
Proportion of subjects with first complete closure of target wound within 45 days 41.3% 28.9% (0.8, 25.6) By EB subtype* RDEB (n=175) 44.0% 26.2% (3.9, 31.6) DDEB (n=20) 50.0% 50.0% (-47.8, 47.8) JEB (n=26) 18.2% 26.7% (-40.4, 23.5) Proportion of subjects with first complete closure of target wound within 90 days 50.5% 43.9% (-6.2, 20.0) -
16 HOW SUPPLIED/STORAGE AND HANDLING
FILSUVEZ (birch triterpenes) topical gel, 10% (w/w) is a colorless to slightly yellowish, opalescent, non-aqueous gel and is supplied in 25 mL white aluminum tubes containing 23.4 grams of gel per tube (NDC: 76431-310-01).
Each sterile tube is for one-time use only. Once opened, the product should be used immediately and discarded after use.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Administration Instructions
Advise patients (and/or their caregivers or guardians) of the following [see Dosage and Administration (2)]:
- Wash hands before and after applying FILSUVEZ or wear gloves for application.
- Apply a 1 mm layer of FILSUVEZ to the affected wound surface. Do not rub in the gel. Cover the wound with a sterile non-adhesive wound dressing. Alternatively, apply a generous layer of FILSUVEZ directly to the dressing so that the gel is in direct contact with the wound.
- Apply FILSUVEZ to cleansed wounds at dressing changes until the wound is healed.
- FILSUVEZ is for topical use only. FILSUVEZ is not for ophthalmic use and should not be applied to mucous membranes. In case of accidental contact, irrigate eyes with water.
- The sterile tube of topical gel is for one-time use only. Once the tube is opened, use the product immediately. Discard the tube after use, even if there is some topical gel left.
Hypersensitivity Reactions
- Inform patients that local hypersensitivity and skin reactions, including urticaria and dermatitis, have been reported in patients treated with FILSUVEZ. If signs and symptoms of local hypersensitivity or skin reactions occur, instruct patients to discontinue FILSUVEZ immediately and contact their healthcare provider [see Warnings and Precautions (5.1)].
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
FILSUVEZ (fill-sue-vez)
(birch triterpenes) topical gelThis Patient Information has been approved by the U.S. Food and Drug Administration Issued: 12/2023 Important information: FILSUVEZ is for use on the skin (topical use) only. Do not use FILSUVEZ in your eyes, mouth, vagina or anus. What is FILSUVEZ?
FILSUVEZ is a prescription medicine used on the skin to treat wounds that may happen with dystrophic and junctional epidermolysis bullosa (EB) in adults and children 6 months of age and older.
It is not known if FILSUVEZ is safe and effective in children younger than 6 months of age.Do not use FILSUVEZ if you are allergic to any of its ingredients. See the end of this leaflet for a complete list of ingredients in FILSUVEZ. Before using FILSUVEZ, tell your healthcare provider about all of your medical conditions, including if you: - are pregnant or plan to become pregnant. It is not known if FILSUVEZ will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if FILSUVEZ passes into your breast milk.
How should I use FILSUVEZ?
See the detailed "Instructions for Use" that comes with FILSUVEZ for information on how to apply FILSUVEZ.- Use FILSUVEZ exactly as your healthcare provider tells you to use it.
- Clean wounds before applying FILSUVEZ as instructed by your healthcare provider.
- Wash hands before applying FILSUVEZ or wear gloves.
- Apply FILSUVEZ at each dressing change until the wound is healed.
- Check the expiration date on the FILSUVEZ tube. Do not use FILSUVEZ if the expiration date has passed. Call your pharmacist or healthcare provider for instructions.
- The tube of FILSUVEZ is for one-time use only. After the tube has been opened, apply the gel right away. Throw away any remaining gel and the tube after use.
- Wash hands after applying FILSUVEZ and caring for wounds.
- Do not use around or get FILSUVEZ in the eyes, or mucous membrane areas examples are mouth, vagina or anus.
- If you get FILSUVEZ in your eyes or mucous membrane area, rinse with clean water right away. Contact your healthcare provider if you have any discomfort.
- If the wounds you are treating with FILSUVEZ become infected, stop treatment and contact your healthcare provider. Signs or symptoms of infection may include the wound becoming red, warm, swollen, painful or drains yellow or greenish fluid (pus).
What are the possible side effects of FILSUVEZ?
FILSUVEZ may cause serious side effects including:- Allergic reactions. Allergic reactions and skin reactions to FILSUVEZ may include the following symptoms: red itchy bumps (hives), skin rash, redness or itching. If you get any of these symptoms, stop using FILSUVEZ right away and call your healthcare provider.
These are not all the possible side effects of FILSUVEZ.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store FILSUVEZ? - Store FILSUVEZ at room temperature between 68°F to 77°F (20°C to 25°C). Do not freeze.
- FILSUVEZ is for one-time use only. Throw away (dispose of) any remaining FILSUVEZ and the tube right away after use in household trash or through a drug take-back option, if available. Go to www.fda.gov/drugdisposal for more information on drug disposal.
General information about the safe and effective use of FILSUVEZ
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use FILSUVEZ for a condition for which it was not prescribed. Do not give FILSUVEZ to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about FILSUVEZ that is written for healthcare professionals.What are the ingredients in FILSUVEZ?
Active ingredient: birch triterpenes
Inactive ingredients: refined sunflower oil
Manufactured by: Lichtenheldt GmbH, Pharmazeutische Fabrik, Werk 1, Industriestr. 7-11, 23812 Wahlstedt, Germany.
For more information, call 1-855-303-2347 or go to www.filsuvez.com -
INSTRUCTIONS FOR USE FILSUVEZ (fill-sue-vez) (birch triterpenes) topical gel
Important information: FILSUVEZ is for use on the skin (topical use) only. Do not use FILSUVEZ in or around your eyes or mucous membranes (for example, the mouth, vagina, or anus).
This Instructions for Use contains information on how to apply FILSUVEZ. Read this Instructions for Use before you start using FILSUVEZ. Ask your healthcare provider if you have any questions.

Important Information You Need to Know Before Applying FILSUVEZ
- Use FILSUVEZ exactly as your healthcare provider tells you to use it.
- Apply FILSUVEZ directly to the wound or to the dressing surface that will be in contact with the wound.
- Clean wounds before applying FILSUVEZ as instructed by your healthcare provider.
- Apply FILSUVEZ with a clean or gloved hand.
- Apply FILSUVEZ at each dressing change until the wound is completely healed.
- Do not get FILSUVEZ in or apply around the eyes, or mucous membranes (For example, mouth, vagina, or anus). If you get FILSUVEZ in your eyes or mucous membranes, rinse with clean water right away. Contact your healthcare provider if you have any discomfort.
- Wash hands after applying FILSUVEZ and caring for wounds.
- The tube of FILSUVEZ is for one-time use only. After the tube has been opened, apply the FILSUVEZ gel right away. Throw away any remaining gel and the tube after use.
- Check the expiration date on the FILSUVEZ tube. Do not use FILSUVEZ if the expiration date has passed. Call your pharmacist or healthcare provider for instructions.
Gather your supplies
- a new tube of FILSUVEZ
- gloves (if using)
- sterile, non-adhesive dressing
Applying FILSUVEZ
- Wash your hands with soap and water or wear gloves.
- Open a new tube of FILSUVEZ. Apply the gel right away.
- You can apply FILSUVEZ in 2 ways, either directly to the wound or to the non-adhesive dressing surface that will be in contact with the wound.
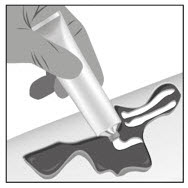
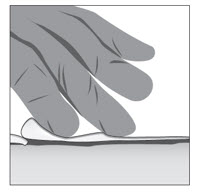
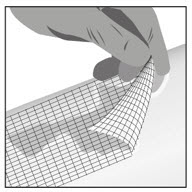
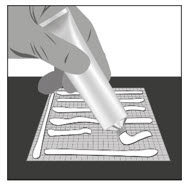
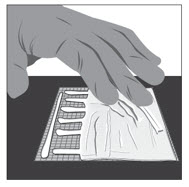
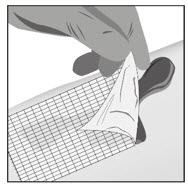
Option 1: Apply FILSUVEZ directly to wound.- Apply a 1 mm layer of FILSUVEZ to the wound (See Step 1). Cover the entire wound surface with a 1 mm layer of FILSUVEZ (Step 2). Do not rub in the gel. Cover the wound with a sterile, non-adhesive wound dressing (see Step 3).
- Apply a generous layer of FILSUVEZ directly to the sterile, non-adhesive dressing (see Step 1). Cover the entire non-adhesive dressing with FILSUVEZ (Step 2). Cover the wound with the dressing so that the gel is in direct contact with the wound (see Step 3). Secure the non-adhesive wound dressing as directed by your healthcare provider .
How should I store FILSUVEZ?
Store FILSUVEZ at room temperature between 68°F to 77°F (20°C to 25°C). Do not freeze.
Keep FILSUVEZ and all medicines out of the reach of children.
How should I throw away (dispose of) FILSUVEZ?
Throw away (dispose of) any remaining FILSUVEZ and the tube right away after use in household trash or through a drug take-back option, if available. Go to www.fda.gov/drugdisposal for more information on drug disposal.
Manufactured by: Lichtenheldt GmbH, Pharmazeutische Fabrik, Werk 1, Industriestr. 7-11, 23812 Wahlstedt, Germany
For more information, call 1-855-303-2347 or go to www.filsuvez.com
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Approved: December-2023
-
PRINCIPAL DISPLAY PANEL - 23.4 g Tube Carton
NDC: 76431-310-01
Rx Only
Filsuvez® (birch triterpenes)
topical gel, 10%Topical use only.
Net content 23.4 g
Filsuvez®
(birch triterpenes) topical gel
-
INGREDIENTS AND APPEARANCE
FILSUVEZ
birch triterpenes gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 76431-310 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength birch triterpenes (UNII: BX09B0RQR0) (birch triterpenes - UNII:BX09B0RQR0) birch triterpenes 100 mg in 1 g Inactive Ingredients Ingredient Name Strength sunflower oil (UNII: 3W1JG795YI) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 76431-310-01 1 in 1 CARTON 02/22/2024 06/01/2028 1 23.4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215064 02/22/2024 06/01/2028 Labeler - Amryt Pharmaceuticals DAC (985632604) Establishment Name Address ID/FEI Business Operations Eurofins BioPharma Product Testing Hamburg GmbH 341475834 ANALYSIS(76431-310) Establishment Name Address ID/FEI Business Operations Amryt GmbH 329468685 API MANUFACTURE(76431-310) , ANALYSIS(76431-310) , LABEL(76431-310) , PACK(76431-310) Establishment Name Address ID/FEI Business Operations CIP Chemisches Institut Pforzheim GmbH 329345185 ANALYSIS(76431-310) Establishment Name Address ID/FEI Business Operations BioChem Labor für biologische und chemische Analytik GmbH 318354230 ANALYSIS(76431-310) Establishment Name Address ID/FEI Business Operations Nuvisan GmbH 341633232 ANALYSIS(76431-310) Establishment Name Address ID/FEI Business Operations Lichtenheldt GmbH 316598945 ANALYSIS(76431-310) , LABEL(76431-310) , MANUFACTURE(76431-310) , PACK(76431-310) Establishment Name Address ID/FEI Business Operations Lichtenheldt GmbH 506712962 ANALYSIS(76431-310) Establishment Name Address ID/FEI Business Operations Synergy Health Radeberg GmbH 330473398 sterilize(76431-310) Establishment Name Address ID/FEI Business Operations Pharma Packaging Solutions (Carton Service, Inc.) 928861723 pack(76431-310)
Trademark Results [FILSUVEZ]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 FILSUVEZ 88470837 not registered Live/Pending |
Amryt Research Ltd 2019-06-12 |
 FILSUVEZ 79329353 not registered Live/Pending |
Amryt Research Ltd 2021-11-17 |
 FILSUVEZ 79328937 not registered Live/Pending |
Amryt Research Ltd 2021-11-17 |
 FILSUVEZ 79322377 not registered Live/Pending |
Amryt Research Ltd 2021-08-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.