CETIRIZINE HYDROCHLORIDE tablet
Cetirizine Hydrochloride by
Drug Labeling and Warnings
Cetirizine Hydrochloride by is a Otc medication manufactured, distributed, or labeled by Drug Ocean LLC, Unique Pharmaceutical Laboratories. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredients
- Uses
- Warnings
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- When using this product
- STOP USE
- If pregnant or breast-feeding:
- Keep out of reach of children.
-
Directions
Adults and children 6
years and over
1 to 2 tablets once daily depending upon severity of symptoms; do not take more than 2 tablets in 24 hours. Adults 65 years and over 1 tablet1 tablet once a day; do not take more than 1 tablet in 24 hours. Children under 6 years of age Ask a doctor Consumers with liver or kidney disease Ask a doctor - Other Information
- Inactive Ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
-
Cetirizine Hydrochloride Tablets 5 mg Container Label
DRUG OCEAN NDC: 70985-001-01
Original Prescription Strength
Cetirizine Hydrochloride
Tablets USP5 mg
100 Tablets
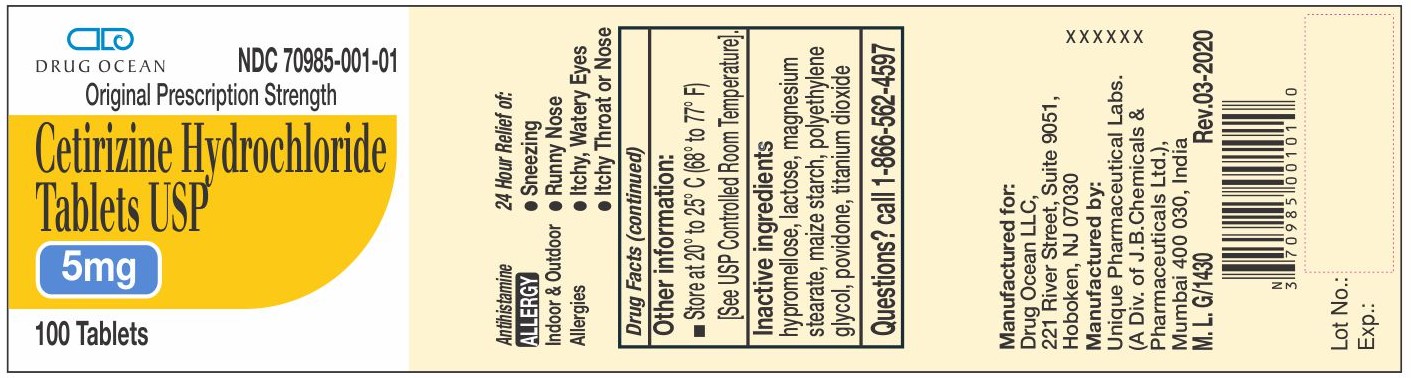
-
INGREDIENTS AND APPEARANCE
CETIRIZINE HYDROCHLORIDE
cetirizine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 70985-001 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white (White) Score no score Shape BULLET (Barrel Shaped) Size 7mm Flavor Imprint Code CTN;5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70985-001-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 11/08/2016 2 NDC: 70985-001-02 500 in 1 BOTTLE; Type 0: Not a Combination Product 11/08/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077829 11/08/2016 Labeler - Drug Ocean LLC (080381835) Registrant - Unique Pharmaceutical Laboratories (917165052) Establishment Name Address ID/FEI Business Operations Unique Pharmaceutical Laboratories 650434645 MANUFACTURE(70985-001)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.