COXIBA- deracoxib tablet, chewable
Coxiba by
Drug Labeling and Warnings
Coxiba by is a Animal medication manufactured, distributed, or labeled by ASPEN VETERINARY RESOURCES®, LTD, FELIX PHARMACEUTICALS PRIVATE LIMITED. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Caution:
-
Description:
CoxibaTM (deracoxib) Chewable Tablets is a non-narcotic, non-steroidal anti-inflammatory drug (NSAID) of the coxib class. Coxiba Chewable Tablets are round, light brown colored, chewable tablets that contain deracoxib formulated with beefy flavoring. The molecular weight of deracoxib is 397.38. The empirical formula is C17-H14-F3- N3-O3-S. Deracoxib is 4-[3-(difluoromethyl)-5-(3-fluoro-4-methoxyphenyl)-1H-pyrazole- 1-yl] benzenesulfonamide, and can be termed a diaryl substituted pyrazole. The structural formula is:
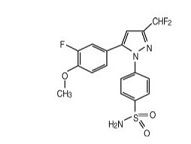
-
Clinical Pharmacology
Mode of Action:
Coxiba Chewable Tablets are a member of the coxib class of non-narcotic, nonsteroidal, cyclooxygenase-inhibiting anti-inflammatory drugs for the control of postoperative pain and inflammation associated with orthopedic and dental surgery and for the control of pain and inflammation associated with osteoarthritis in dogs.
Data indicate that deracoxib inhibits the production of PGE1 and 6-keto PGF1 by its inhibitory effects on prostaglandin biosynthesis.1 Deracoxib inhibited COX-2 mediated PGE2 production in LPS-stimulated human whole blood.2
Cyclooxygenase-1 (COX-1) is the enzyme responsible for facilitating constitutive physiological processes (e.g., platelet aggregation, gastric mucosal protection, renal perfusion).3 Cyclooxygenase-2 (COX-2) is responsible for the synthesis of inflammatory mediators.4 Both COX isoforms are constitutively expressed in the canine kidney.5 At doses of 2-4 mg/kg/day, deracoxib tablets do not inhibit COX-1 based on in vitro studies using cloned canine cyclooxygenase.6 The clinical relevance of this in vitro data has not been shown.
Although the plasma terminal elimination half-life for deracoxib tablets is approximately 3 hours, a longer duration of clinical effectiveness is observed.
Summary pharmacokinetics of deracoxib tablets are listed in Table 1.
Table 1: Pharmacokinetics of Deracoxib Parameter Value Tmaxa
2 hours
Oral Bioavailability (F)a
> 90% at 2 mg/kg
Terminal elimination half-lifeb
3 hours at 2-3 mg/kg
19 hours at 20 mg/kg
Systemic Clearanceb
~ 5 ml/kg/min at 2 mg/kg
~ 1.7 ml/kg/min at 20 mg/kg
Volume of Distributionc
~ 1.5 L/kg
Protein bindingd
> 90%
aValues obtained following a single 2.35 mg/kg dose
bEstimates following IV administration of deracoxib as an aqueous solution
cBased upon a dose of 2 mg/kg of deracoxib
d Based upon in vitro plasma concentrations of 0.1, 0.3, 1.0, 3.0, 10.0 μg/mlNon-linear elimination kinetics are exhibited at doses above 8 mg/kg/day, at which competitive inhibition of constitutive COX-1 may occur.
Deracoxib is not excreted as parent drug in the urine. The major route of elimination of deracoxib is by hepatic biotransformation producing four major metabolites, two of which are characterized as products of oxidation and o-demethylation. The majority of deracoxib is excreted in feces as parent drug or metabolite.
Large intersubject variability was observed in drug metabolite profiles of urine and feces. No statistically significant differences between genders were observed.
-
Indications and Usage:
Always provide “Information for Dog Owners” Sheet with prescription. Carefully consider the potential benefits and risk of Coxiba Chewable Tablets and other treatment options before deciding to use Coxiba Chewable Tablets. Use the lowest effective dose for the shortest duration consistent with individual response.
-
Dosage and Administration
Osteoarthritis Pain and Inflammation: 0.45 – 0.91 mg/lb/day (1 to 2 mg/kg/ day) as a single daily dose, as needed. Dogs needing a dose of less than 12.5 mg can only be accurately dosed through use of the 12 mg tablet, which can be broken in half to provide 6 mg. Do not attempt to accurately dose smaller dogs through the use of breaking larger tablets. Inaccurate dosing may result in adverse drug events (see Adverse Reactions, Animal Safety, and Post-Approval Experience).
-
Dosage and Administration
Postoperative Orthopedic Pain and Inflammation: 1.4 – 1.8 mg/lb/day (3 to 4 mg/kg/day) as a single daily dose, as needed, not to exceed 7 days of administration.
Dogs needing a dose of less than 12.5 mg can only be accurately dosed through use of the 12 mg tablet, which can be broken in half to provide 6 mg. Do not attempt to accurately dose smaller dogs through the use of breaking larger tablets. Inaccurate dosing may result in adverse drug events (see Adverse Reactions, Animal Safety, and Post-Approval Experience).
-
Dosage and Administration
Postoperative Dental Pain and Inflammation: 0.45 – 0.91 mg/lb/day (1 to 2 mg/kg/day) as a single daily dose, for 3 days. The first dose should be given approximately 1 hour prior to dental surgery and subsequent doses should be given daily for up to two additional treatments.
Dogs needing a dose of less than 12.5 mg can only be accurately dosed through use of the 12 mg tablet, which can be broken in half to provide 6 mg. Do not attempt to accurately dose smaller dogs through the use of breaking larger tablets. Inaccurate dosing may result in adverse drug events (see Adverse Reactions, Animal Safety, and Post-Approval Experience).
Since deracoxib tablet bioavailability is greatest when taken with food, postprandial administration is preferable. However, deracoxib tablets have been shown to be effective under both fed and fasted conditions; therefore, they may be administered in the fasted state if necessary. For postoperative orthopedic and dental pain, administer Coxiba Chewable Tablets prior to the procedure. Tablets are scored and dosage should be calculated in half-tablet increments. In clinical practice it is recommended to adjust the individual patient dose while continuing to monitor the dog's status until a minimum effective dose has been reached.
- Contraindications:
-
Warnings:
Not for use in humans. Keep this and all medications out of reach of children. Consult a physician in case of accidental ingestion by humans. For use in dogs only. Do not use in cats.
Dogs needing a dose of less than 12.5 mg can only be accurately dosed through use of the 12 mg tablet, which can be broken in half to provide 6 mg. Do not attempt to accurately dose smaller dogs through the use of breaking larger tablets. Inaccurate dosing may result in adverse drug events (see Adverse Reactions, Animal Safety, and Post-Approval Experience).
All dogs should undergo a thorough history and physical examination before the initiation of NSAID therapy. Appropriate laboratory tests to establish hematological and serum biochemical baseline data prior to, and periodically during, administration of any NSAID is recommended. Owners should be advised to observe for signs of potential drug toxicity (see Adverse Reactions, Animal Safety and Post-Approval Experience) and be given an “Information for Dog Owners” Sheet.
Keep Coxiba in a secure location out of reach of dogs, cats, and other animals to prevent accidental ingestion or overdose.
-
Precautions:
Dogs needing a dose of less than 12.5 mg can only be accurately dosed through use of the 12 mg tablet, which can be broken in half to provide 6 mg. Do not attempt to accurately dose smaller dogs through the use of breaking larger tablets. Inaccurate dosing may result in adverse drug events (see Adverse Reactions, Animal Safety, and Post-Approval Experience).
Since NSAIDs possess the potential to produce gastrointestinal ulceration and/or perforation, concomitant use of Coxiba Chewable Tablets with other anti-inflammatory drugs, such as NSAIDs or corticosteroids, should be avoided. As a class, NSAIDs may be associated with gastrointestinal, renal and hepatic toxicity. The following collective group of clinical signs has been reported with some serious gastrointestinal events, in decreasing order of reported frequency: anorexia, tachycardia, tachypnea, pyrexia, ascites, pale mucous membranes, dyspnea. In some cases, circulatory shock, collapse and cardiac arrest have also been reported. Sensitivity to drug-associated adverse events varies with the individual patient. Dogs that have experienced adverse reactions from one NSAID may experience adverse reactions from another NSAID.
Patients at greatest risk for adverse events are those that are dehydrated, on concomitant diuretic therapy, or those with existing renal, cardiovascular, and/ or hepatic dysfunction. Plasma levels of deracoxib may increase in a greater than dose- proportional fashion above 8 mg/kg/day. Deracoxib tablets have been safely used during field studies in conjunction with other common medications, including heartworm preventatives, anthelmintics, anesthetics, pre-anesthetic medications, and antibiotics. If additional pain medication is needed after a daily dose of Coxiba Chewable Tablets, a non- NSAID/non-corticosteroid class of analgesic may be necessary. It is not known whether dogs with a history of hypersensitivity to sulfonamide drugs will exhibit hypersensitivity to Coxiba Chewable Tablets. The safe use of deracoxib tablets in dogs younger than 4 months of age, dogs used for breeding, or in pregnant or lactating dogs has not been evaluated.
NSAIDs may inhibit the prostaglandins which maintain normal homeostatic function. Such anti-prostaglandin effects may result in clinically significant disease in patients with underlying or pre-existing disease that has not been previously diagnosed. Appropriate monitoring procedures should be employed during all surgical procedures. The use of parenteral fluids during surgery should be considered to decrease potential renal complications when using NSAIDs perioperatively. Concurrent administration of potentially nephrotoxic drugs should be carefully approached.
The use of concomitantly protein-bound drugs with deracoxib tablets has not been studied in dogs. Commonly used protein-bound drugs include cardiac, anticonvulsant and behavioral medications. The influence of concomitant drugs that may inhibit metabolism of deracoxib tablets has not been evaluated. Drug compatibility should be monitored in patients requiring adjunctive therapy. Consider appropriate washout times when switching from one NSAID to another or when switching from corticosteroid use to NSAID use. -
Animal Safety:
In a 6-month study, dogs were dosed with deracoxib at 0, 2, 4, 6, 8 and 10 mg/kg with food once daily for 6 consecutive months. There were no abnormal feces, and no abnormal findings on clinical observations, food and water consumption, body weights, physical examinations, ophthalmoscopic evaluations, macroscopic pathological examinations, hematology, or buccal bleeding time. Urinalysis results showed hyposthenuria (specific gravity <1.005) and polyuria in one male and one female in the 6 mg/kg group after 6 months of treatment. After 6 months of treatment, the mean BUN values for dogs treated with 6, 8, or 10 mg/kg/day were 30.0, 35.3, and 48.2 mg/dL respectively. No effects were seen on any other clinical chemistry parameters, including other variables associated with renal physiology (serum creatinine, serum electrolytes, and urine sediment evaluation). Dose dependent focal renal tubular degeneration/regeneration was seen in some dogs treated at 6, 8, and 10 mg/kg/day. Focal renal papillary necrosis was seen in 3 dogs dosed at 10 mg/kg/day and in one dog dosed at 8 mg/kg/day. No renal lesions were seen at the label doses of 2 and 4 mg/kg/day. There was no evidence of gastrointestinal, hepatic, or hematopoietic pathology at any of the doses tested.
In a laboratory study, healthy young dogs were dosed with deracoxib tablets once daily, within 30 minutes of feeding, at doses of 0, 4, 6, 8, and 10 mg/kg body weight for 21 consecutive days. No adverse drug events were reported. There were no abnormal findings reported for clinical observations, food and water consumption, body weights, physical examinations, ophthalmic evaluations, organ weights, macroscopic pathologic evaluation, hematology, urinalyses, or buccal mucosal bleeding time. In the clinical chemistry results there was a statistically significant (p<0.0009) dose-dependent trend toward increased levels of blood urea nitrogen (BUN). Mean BUN values remained within historical normal limits at the label dose. No effects on other clinical chemistry values associated with renal function were reported. There was no evidence of renal, gastrointestinal, hepatic or biliary lesions noted during gross necropsy. Renal histopathology revealed trace amounts of tubular degeneration/regeneration in all dose groups including placebo, but no clear dose relationship could be determined. There was no histopathologic evidence of gastrointestinal, hepatic or biliary lesions.
In another study, micronized deracoxib in gelatin capsules was administered once daily to healthy young dogs at doses of 10,25, 50, and 100 mg/kg body weight for periods up to 14 consecutive days. Food was withheld prior to dosing. Non-linear elimination kinetics occurred at all doses. At doses of 25, 50, and 100 mg/kg, reduced body weight, vomiting, and melena occurred. Necropsy revealed gross gastrointestinal lesions in dogs from all dose groups. The frequency and severity of the lesions increased with escalating doses. At 10 mg/kg, moderate diffuse congestion of gut associated lymphoid tissues (GALT) and erosions/ulcers in the jejunum occurred. At 100 mg/kg, all dogs exhibited gastric ulcers and erosions/ulcerations of the small intestines. There were no hepatic or renal lesions reported at any dose in this study.
In a 13-week study, deracoxib in gelatin capsules was administered to healthy dogs at doses of 0, 2, 4, and 8 mg/kg/day. No test-article related changes were identified in clinical observations, physical exams, or any of the other parameters measured. One dog in the 8 mg/kg dose group died from bacterial septicemia secondary to a renal abscess. The relationship between deracoxib administration and the renal abscess is not clear.
- Effectiveness:
-
Osteoarthritis Pain and Inflammation Field Study:
Two hundred and nine (209) client-owned dogs with clinical and radiographic signs of osteoarthritis of at least one appendicular joint were enrolled in this study. A total of 194 dogs were included in the safety evaluation and a total of 181 dogs were included in the effectiveness evaluation. The effectiveness of deracoxib tablets in the control of pain and inflammation associated with osteoarthritis was demonstrated in a placebo-controlled, masked study evaluating the anti-inflammatory and analgesic effects of deracoxib tablets. Tablets were administered by the owner at approximately 1-2 mg/kg/day for forty-three (43) consecutive days.
In general, statistically significant (p≤ 0.05) differences in favor of deracoxib were seen for force plate parameters (vertical impulse area, peak vertical force) and owner evaluations (quality of life, lameness and overall level of activity).
The results of this field study demonstrate that deracoxib tablets, when administered at 1-2 mg/kg/day for 43 days, are effective for the control of pain and inflammation associated with osteoarthritis. -
Adverse Reactions:
Deracoxib was well tolerated and the incidence of clinical adverse reactions was comparable in deracoxib and placebo-treated animals. A total of 209 dogs of 41 breeds, 1-14 years old, weighing 17-177 lbs were included in the field safety analysis. The following table shows the number of dogs displaying each adverse reaction.
Abnormal Health Findings in the Osteoarthritis Field Study1
Clinical Observation
Deracoxib tablets
N= 105
Placebo
N = 104Vomiting
3
4Diarrhea/soft stool
3
2Weight loss
1
0Abdominal pain (splinting)
0
1Seizure
1
0Lethargy
0
1Pyoderma/Dermatitis
2
0Unilateral conjuctivitis
1
0Scleral injection
0
1Hematuria/UTI
1
0Splenomegaly*
1
0Grade II murmur systolic
1
01Dogs may have experienced more than one adverse reaction during the study.
* This dog was less active and eating less on enrollment, with elevated WBC, amylase, and AST and died 1 month after exiting the study. The dog was withdrawn from the study on Day 17 with anorexia, lethargy and a suspicion of diarrhea. Follow-up laboratory analyses revealed hypoalbuminemia, hyperphosphatemia, elevated AST and decreased BUN. Follow-up treatment included other anti-inflammatories and antibiotics.
Complete blood count, serum chemistry, and buccal bleeding time analysis were conducted at the beginning and end of the trial. Mean values of all CBC and chemistry results for both deracoxib and placebo-treated dogs were within normal limits.
There was no statistically significant difference in the buccal bleeding time between deracoxib and placebo-treated dogs before or after the study, and all results remained within normal limits (less than 5 minutes). The results of this field study demonstrate that deracoxib is safe and effective for the control of pain and inflammation associated with osteoarthritis in dogs.
During this trial, dogs were safely treated with a variety of commonly used medications, including antibiotics, anti-parasiticides, topical flea adulticides and thyroid supplements.
The results of this field study demonstrate that deracoxib tablets are well tolerated when administered at 1-2 mg/kg/day for up to 43 days for the control of pain and inflammation associated with osteoarthritis. -
Postoperative Orthopedic Pain and Inflammation Field Study:
In this study, 207 dogs admitted to veterinary hospitals for repair of a cranial cruciate injury were randomly administered deracoxib tablets or a placebo. Drug administration started the evening before surgery and continued once daily for 6 days postoperatively. Effectiveness was evaluated in 119 dogs and safety was evaluated in 207 dogs. Statistically significant differences in favor of deracoxib tablets were found for lameness at walk and trot, and pain on palpation values at all post-surgical time points. The results of this field study demonstrate that deracoxib tablets, when administered daily for 7 days are effective for the control of postoperative pain and inflammation associated with orthopedic surgery.
-
Adverse Reactions:
A total of 207 dogs of forty-three (43) different breeds, 1-15 years old, weighing 7-141 lbs were included in the field safety analysis. The following table shows the number of dogs displaying each adverse reaction.
Abnormal Health Findings in The Postoperative Orthopedic Pain Field Study1
Clinical Observation
Deracoxib tablets
N = 105
Placebo
N = 102Vomiting
11
6Diarrhea
6
7Hematochezia
4
0Melena
0
1Anorexia
0
4Incision site lesion (drainage, oozing)
11
6Non-incision skin lesions (moist dermatitis, pyoderma)
2
0Otitis externa
2
0Positive joint culture
1
0Phlebitis
1
0Hematuria
2
0Conjunctivitis
1
2Splenomegaly
1
0Hepatomegaly
1
0Death
0
11 Dog may have experienced more than one adverse reaction during the study.
This table does not include one dog that was dosed at 16.92 mg/kg/day for the study duration. Beginning on the last day of treatment, this dog experienced vomiting, diarrhea, increased water intake and decreased appetite. Hematology and clinical chemistry values were unremarkable. The dog recovered uneventfully within 3 days of cessation of dosing.
Incisional drainage was most prevalent in dogs enrolled at a single study site. There were no statistically significant changes in the mean values for hepatic or renal clinical pathology indices between deracoxib tablet and placebo treated dogs. Four deracoxib tablet-treated dogs and two placebo-treated dogs exhibited elevated bilirubin during the dosing phase. One deracoxib tablet-treated dog exhibited elevated ALT, BUN and total bilirubin and a single vomiting event. None of the changes in clinical pathology values were considered clinically significant.
The results of this clinical study demonstrate that deracoxib tablets, when administered daily for 7 days to control postoperative orthopedic pain and inflammation in dogs, are well tolerated.
-
Postoperative Dental Pain and Inflammation Field Study:
In this study, 62 dogs admitted to veterinary hospitals for dental extractions were randomly administered deracoxib tablets or a placebo. Drug administration started approximately 1 hour before surgery and continued once daily for 2 days postoperatively. Effectiveness was evaluated in 57 dogs and safety was evaluated in 62 dogs. There was a statistically significant reduction (p=0.0338) in the proportion of dogs that required rescue therapy to control post-surgical pain in the deracoxib treated group compared to the placebo control group. Pain assessors used a modification of the Glasgow Composite Pain Scale (mGCPS) to assess pain.7 A dog was rescued if it scored ≥ 4 on the combined mGCPS variables of Posture/Activity, Demeanor, Response to Touch, and Vocalization, or if the investigator determined at any time that pain intervention was needed. The results of this field study demonstrate that deracoxib, when administered once daily for 3 days, is effective for the control of postoperative pain and inflammation associated with dental surgery.
-
Adverse Reactions:
A total of 62 male and female dogs of various breeds, 1.5-16 years old, were included in the field safety analysis. The following table shows the number of dogs displaying each adverse reaction. Digestive tract disorders (diarrhea and vomiting) and systemic disorders (abnormal clinical chemistry results) were the most frequently reported findings. There were no distinct breed, age, or sex predilections for adverse reactions that were reported. No dogs were withdrawn from the study due to the occurrence of an adverse reaction.
Abnormal Health Findings in the Dental Pain Field Study1 Clinical Observation
Deracoxib Tablets
N=31
Placebo
N=31Vomiting
4
1Diarrhea/soft stool
3
1Regurgitation
0
2Increased AST2
3
0Increased ALT2
1
0Hematuria
1
0Leukocytosis
1
1Neutrophilia
1
1Lameness
1
0Facial swelling
0
1Tachycardia
0
11Dogs may have experienced more than one adverse reaction during the study.
2Included animals with results over 2x the high normal. -
Post Approval Experience (Rev. 2010):
The following adverse events are based on post-approval adverse drug experience reporting. Not all adverse reactions are reported to FDA CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using this data. The following adverse events are grouped by body system and are presented in decreasing order of reporting frequency.
Gastrointestinal: vomiting, diarrhea, hypoalbuminemia, melena, hematochezia, elevated amylase/lipase, hematemesis, abdominal pain, peritonitis, decreased or increased total protein and globulin, gastrointestinal perforation, gastrointestinal ulceration, hypersalivation.
General: anorexia, depression/lethargy, weight loss, weakness, fever, dehydration
Hepatic: elevated liver enzymes, hyperbilirubinemia, icterus, ascites, decreased BUN
Hematologic: anemia, leukocytosis, leukocytopenia, thrombocytopenia
Neurologic: seizures, ataxia, recumbency, trembling, confusion, collapse, hind limb paresis, nystagmus, proprioceptive disorder, vestibular signs
Behavioral: nervousness, hyperactivity, aggression, apprehension
Urologic: elevated BUN/creatinine, polydipsia, polyuria, hyper-phosphatemia, hematuria, low urine specific gravity, urinary incontinence, renal failure, urinary tract infection
Dermatologic: pruritus, erythema, urticaria, moist dermatitis, facial/muzzle edema, dermal ulceration/necrosis
Respiratory: panting, dyspnea, epistaxis, coughing
Cardiovascular: tachycardia, heart murmur, bradycardia, arrest
Sensory: Vestibular signs, glazed eyes, uveitis.
Ophthalmic: blindness, mydriasis, conjunctivitis, keratoconjunctivitis sicca, uveitis.
In some cases, death has been reported as an outcome of the adverse events listed above.
To report suspected adverse drug events, for technical assistance or to obtain a copy of the Safety Data Sheet, contact manufacturer at 1-833-571-1525. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae. -
Chewable Tablets
Information for Dog Owners:
Coxiba Chewable Tablets, like other drugs of its class, is not free from adverse reactions. Owners should be advised of the potential for adverse reactions and be informed of the clinical signs associated with drug intolerance. Adverse reactions may include vomiting, diarrhea, decreased appetite, dark or tarry stools, increased water consumption, increased urination, anemia, yellowing of gums, skin or white of the eye due to jaundice, lethargy, incoordination, seizure, or behavioral changes.
Serious adverse reactions associated with this drug class can occur without warning and in some cases result in death (see Warnings, Post-Approval Experience and Adverse Reactions). Owners should be advised to discontinue Coxiba Chewable Tablets therapy and contact their veterinarian immediately if signs of intolerance are observed.
The vast majority of patients with drug related adverse reactions have recovered when the signs are recognized, the drug is withdrawn, and veterinary care, if appropriate, is initiated. Owners should be advised of the importance of periodic follow up for all dogs during administration of any NSAID. - Storage Conditions:
-
How Supplied:
Coxiba Chewable Tablets are available as 12 mg, 25 mg, 75 mg and 100 mg round, brownish, half-scored tablets in 30 and 90 count bottles.
NDC Number
Tablet Size
Tablets/Bottle
46066-105-36
12 mg
30
46066-105-37
12 mg
90
46066-106-36
25 mg
30
46066-106-37
25 mg
90
46066-107-36
75 mg
30
46066-107-37
75 mg
90
46066-104-36
100 mg
30
46066-104-37
100 mg
90
-
References:
- 1 Data on file under NADA 141-203
- 2 Data on file under NADA 141-203
- 3 Smith, et al.: "Pharmacological Analysis of Cyclo-oxygenase-1 in Inflammation," Proc. Natl. Acad. Sci. USA (October 1998) 95: 13313-13318, Pharmacology.
- 4 Zhang, et al.: "Inhibition of Cyclo-oxygenase-2 Rapidly Reverses Inflammatory Hyperalgesia and Prostaglandin E2 Production,"JPET, (1997) 283: 1069-1075.
- 5 Verburg, KM et al. "Cox-2 Specific Inhibitors: Definition of a New Therapeutic Concept." Amer J of Therapeutics 8, 49-64, 2001.
- 6 Data on file under NADA 141-203
- 7 Holton, L., Reid, J., Scott, E.M., Pawson, P. and Nolan, A. (2001). Development of a behaviour-based scale to measure acute pain in dogs. Veterinary Record, 148, 525-53.
- SPL UNCLASSIFIED SECTION
-
Information for Dog Owners
CoxibaTM (deracoxib)
Chewable Tablets
For Oral Use in Dogs Only
Do Not Use in CatsInformation for Dog Owners
Coxiba™ (deracoxib) Chewable Tablets are for the control of pain and inflammation due to osteoarthritis or following orthopedic and dental surgery. This summary contains important information about Coxiba Chewable Tablets. You should read this information before starting your dog on Coxiba Chewable Tablets. This sheet is provided only as a summary and does not take the place of instructions from your veterinarian. Talk to your veterinarian if you do not understand any of this information or you want to know more about Coxiba Chewable Tablets.
What is Coxiba Chewable Tablets?
Coxiba Chewable Tablets are a prescription non-steroidal anti-inflammatory drug (NSAID) of the coxib class. They are indicated for the control of postoperative pain and inflammation associated with orthopedic (bone) and dental surgery in dogs and for the control of pain and inflammation (soreness) associated with osteoarthritis in dogs. The tablets are flavored to make administration more convenient.
What kind of results can I expect when my dog takes Coxiba Chewable Tablets for postoperative orthopedic and dental pain and inflammation?
Coxiba Chewable Tablets allow your dog to recover more comfortably by controlling pain and inflammation that follow orthopedic and dental surgery.
The postoperative orthopedic pain dose is a higher dose; administration at this dose should not exceed 7 days total, including the days the patient is administered Coxiba Chewable Tablets while in the hospital.
Control of pain and inflammation may vary from dog to dog.
If Coxiba Chewable Tablets are not given according to your veterinarian's directions, your dog's pain may return.
Consult your veterinarian if your dog appears to be uncomfortable.
What kind of results can I expect when my dog takes Coxiba Chewable Tablets for pain and inflammation due to osteoarthritis?
Osteoarthritis is a painful condition caused by damage to cartilage and other parts of the joint that may result in the following changes or signs in your dog:
Limping or lameness
Decreased activity or exercise (reluctance to stand, climb stairs, jump or run, or difficulty in performing these activities)
Stiffness or decreased movement of joints
While Coxiba Chewable Tablets is not a cure for osteoarthritis, it can control the pain and inflammation of osteoarthritis and improve your dog's mobility. Response may vary from dog to dog but can be quite dramatic. Coxiba Chewable Tablets may need to be given on a periodic basis for the animal's lifetime. Use the lowest dose to provide adequate relief. Always consult with your veterinarian before altering the dose.
What dogs should not take Coxiba Chewable Tablets?
Your dog should not be given Coxiba Chewable Tablets if s/he:
Has had an allergic reaction to deracoxib, the active ingredient in Coxiba Chewable Tablets
Has had an allergic reaction (such as hives, facial swelling, or red or itchy skin) to aspirin or other NSAIDs
Is presently taking aspirin, other NSAIDs, or corticosteroids (unless directed by your veterinarian)
Has bloody stool or vomit
Has a pre-existing kidney or liver condition
Has any condition predisposing to dehydration
Is anorexic (loss of appetite)
Coxiba Chewable Tablets should only be given to dogs. Do not use in cats.
People should not take Coxiba Chewable Tablets. Keep Coxiba Chewable Tablets and all medication out of reach of children.
Call your physician immediately if you accidentally take Coxiba Chewable Tablets.
What to discuss with your veterinarian before giving Coxiba Chewable Tablets?
Tell your veterinarian about:
Any side effects your dog has experienced from Coxiba Chewable Tablets or other NSAIDs
Any digestive upset (vomiting or diarrhea) your dog has had
Any kidney disease your dog has had
Any other medical problems or allergies that your dog has now or has had in the past
All medications that you are giving your dog or plan to give your dog, including those you can get without prescription and
any dietary supplements
If you plan to breed your dog, or if your dog is pregnant or nursing
Talk to your veterinarian about:
The orthopedic or dental surgery your dog will undergo
What tests might be done before surgery is performed or Coxiba Chewable Tablets are prescribed
The signs of pain or inflammation that may occur after surgery
Normal events that can be expected after your dog undergoes surgery
The proper amount of exercise after surgery to aid recovery
The signs of osteoarthritis you have observed (for example, limping or stiffness)
The importance of weight control, physical therapy and exercise in the management of osteoarthritis
How often your dog may need to be examined by your veterinarian
The risks and benefits of using Coxiba Chewable Tablets
How to give Coxiba Chewable Tablets to your dog.
Coxiba Chewable Tablets should be given according to your veterinarian's instructions. Your veterinarian will tell you what amount of Coxiba Chewable Tablets is right for your dog and for how long they should be given. Do not change the way you give Coxiba Chewable Tablets to your dog without first speaking with your veterinarian. Coxiba Chewable Tablets should be given by mouth and may be given with or without food, although with food is preferable.
What are the possible side effects that may occur in my dog during therapy with Coxiba Chewable Tablets?
Coxiba Chewable Tablets may cause some side effects in individual dogs. Serious side effects associated with this drug can occur with or without warning and, in some cases, result in death. The most common side effects associated with Coxiba Chewable Tablets therapy involve the digestive tract (vomiting, decreased appetite and diarrhea). Liver and kidney problems have also been reported. It is important to stop the medication and contact your veterinarian immediately if you think your dog may have a medical problem or side effect while on Coxiba Chewable Tablets. If you have additional questions about possible side effects, talk with your veterinarian or call the manufacturer at 1-833-571-1525.
Look for the following side effects that may indicate that your dog is having a problem with Coxiba Chewable Tablets or may
have another medical problem:
Vomiting
Decrease in appetite
Change in behavior, such as depression, restlessness, aggression or apprehension
Change in bowel movements such as diarrhea or change in stool color (black, tarry or bloody stool)
Change in drinking or urination
Yellowing of gums, skin or whites of the eyes (jaundice)
Can Coxiba Chewable Tablets be given with other medications?
Coxiba Chewable Tablets should not be given with other non-steroidal anti-inflammatory drugs (NSAIDs) or corticosteroids (for example, aspirin, carprofen, etodolac, prednisone).
Tell your veterinarian about all medications that you have given your dog in the past, and any medications that you are planning to give with Coxiba Chewable Tablets. This should include any medications that you can get without a prescription and any dietary supplements. Your veterinarian may want to evaluate the potential for any drug interactions and to assure drug compatibility.
What can I do in case my dog eats more than the prescribed amount of Coxiba Chewable Tablets?
Contact your veterinarian immediately if your dog eats more than the prescribed amount of Coxiba Chewable Tablets.
What else should I know about Coxiba Chewable Tablets?
This sheet provides a summary of information about Coxiba Chewable Tablets. If you have any questions or concerns about Coxiba Chewable Tablets, postoperative orthopedic and dental pain and inflammation, or osteoarthritis, talk to your veterinarian.
As with all prescribed medications, Coxiba Chewable Tablets should only be given to the dog for which they are prescribed. They should be given to your dog only for the condition for which they were prescribed, at the prescribed dose, as directed by your veterinarian. It is important to periodically discuss your dog's response to Coxiba Chewable Tablets at regular checkups. Your veterinarian will best determine if your dog is responding as expected and if your dog should continue receiving Coxiba Chewable Tablets.Keep Coxiba in a secure location out of reach of dogs, cats, and other animals to prevent accidental ingestion or overdose. Use within 90 days of splitting. Approved by FDA under ANADA # 200-704

Manufactured for:
Aspen Veterinary Resource®, LTD., Liberty, MO 64068
pivetalvet.com | P237FPH Rev. 01/23
Made in India Neutral Code No.MP/DRUGS/25/90/2020
-
PRINCIPAL DISPLAY PANEL - 12 mg x 30 count
NDC: 46066-105-36
COXIBATM
(deracoxib)
CHEWABLE TABLETS
For Oral Use in Dogs Only
Do Not Use in Cats
A coxib-class NSAID
12 mg
Caution: Federal law (U.S.) restricts this drug to use by or on the order of a licensed veterinarian.
Approved by FDA under ANADA #200-704
30 Tablets
-
PRINCIPAL DISPLAY PANEL - 12 mg x 90 count
NDC: 46066-105-37
COXIBATM (deracoxib)
CHEWABLE TABLETSFor Oral Use in Dogs Only
Do Not Use in Cats
A coxib-class NSAID12 mg
Caution: Federal law (U.S.) restricts this drug to use by or on the order of a licensed veterinarian.
Approved by FDA under ANADA #200-704
90 Tablets

- PRINCIPAL DISPLAY PANEL - 25 mg x 30 count
-
PRINCIPAL DISPLAY PANEL - 25 mg x 90 count
NDC: 46066-106-37
COXIBATM
(deracoxib) CHEWABLE TABLETS
For Oral Use in Dogs Only
Do Not Use in Cats
A coxib-class NSAID25 mg
Caution: Federal law (U.S.) restricts this drug to use by or on the order of a licensed veterinarian.
Approved by FDA under ANADA #200-704
90 Tablets

-
PRINCIPAL DISPLAY PANEL - 75 mg x 30 count
NDC: 46066-107-36
COXIBATM(deracoxib)
CHEWABLE TABLETSFor Oral Use in Dogs Only
Do Not Use in Cats
A coxib-class NSAID75 mg
Caution: Federal law (U.S.) restricts this drug to use by or on the order of a licensed veterinarian.
Approved by FDA under ANADA #200-704
30 Tablets

-
PRINCIPAL DISPLAY PANEL - 75 mg x 90 count
NDC: 46066-107-37
COXIBATM
(deracoxib)
CHEWABLE TABLETSFor Oral Use in Dogs Only
Do Not Use in Cats
A coxib-class NSAID75 mg
Caution: Federal law (U.S.) restricts this drug to use by or on the order of a licensed veterinarian.
Approved by FDA under ANADA #200-704
90 Tablets

-
PRINCIPAL DISPLAY PANEL - 100 mg x 30 count
NDC: 46066-104-36
COXIBATM(deracoxib)
CHEWABLE TABLETSFor Oral Use in Dogs Only
Do Not Use in Cats
A coxib-class NSAID100 mg
Caution: Federal law (U.S.) restricts this drug to use by or on the order of a licensed veterinarian.
Approved by FDA under ANADA #200-704
30 Tablets
-
PRINCIPAL DISPLAY PANEL - 100 mg x 90 count
NDC: 46066-104-37
COXIBATM
(deracoxib)
CHEWABLE TABLETSFor Oral Use in Dogs Only
Do Not Use in Cats
A coxib-class NSAID100 mg
Caution: Federal law (U.S.) restricts this drug to use by or on the order of a licensed veterinarian.
Approved by FDA under ANADA #200-704
90 Tablets

-
INGREDIENTS AND APPEARANCE
COXIBA
deracoxib tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 46066-105 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DERACOXIB (UNII: VX29JB5XWV) (DERACOXIB - UNII:VX29JB5XWV) DERACOXIB 12 mg Product Characteristics Color BROWN Score 2 pieces Shape ROUND Size 9mm Flavor MEAT Imprint Code 12;F;1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 46066-105-36 30 in 1 BOTTLE 2 NDC: 46066-105-37 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200704 05/25/2022 COXIBA
deracoxib tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 46066-106 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DERACOXIB (UNII: VX29JB5XWV) (DERACOXIB - UNII:VX29JB5XWV) DERACOXIB 25 mg Product Characteristics Color BROWN Score 2 pieces Shape ROUND Size 11mm Flavor MEAT Imprint Code 25;F;1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 46066-106-36 30 in 1 BOTTLE 2 NDC: 46066-106-37 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200704 05/25/2022 COXIBA
deracoxib tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 46066-107 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DERACOXIB (UNII: VX29JB5XWV) (DERACOXIB - UNII:VX29JB5XWV) DERACOXIB 75 mg Product Characteristics Color BROWN Score 2 pieces Shape ROUND Size 16mm Flavor MEAT Imprint Code 75;F;1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 46066-107-36 30 in 1 BOTTLE 2 NDC: 46066-107-37 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200704 05/25/2022 COXIBA
deracoxib tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 46066-104 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DERACOXIB (UNII: VX29JB5XWV) (DERACOXIB - UNII:VX29JB5XWV) DERACOXIB 100 mg Product Characteristics Color BROWN Score 2 pieces Shape ROUND Size 19mm Flavor MEAT Imprint Code 100;F;1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 46066-104-36 30 in 1 BOTTLE 2 NDC: 46066-104-37 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200704 05/25/2022 Labeler - ASPEN VETERINARY RESOURCES®, LTD (627265361) Registrant - FELIX PHARMACEUTICALS PRIVATE LIMITED (985612369)
Trademark Results [Coxiba]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 COXIBA 88273193 not registered Live/Pending |
Aspen Veterinary Resources, Ltd. 2019-01-23 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
