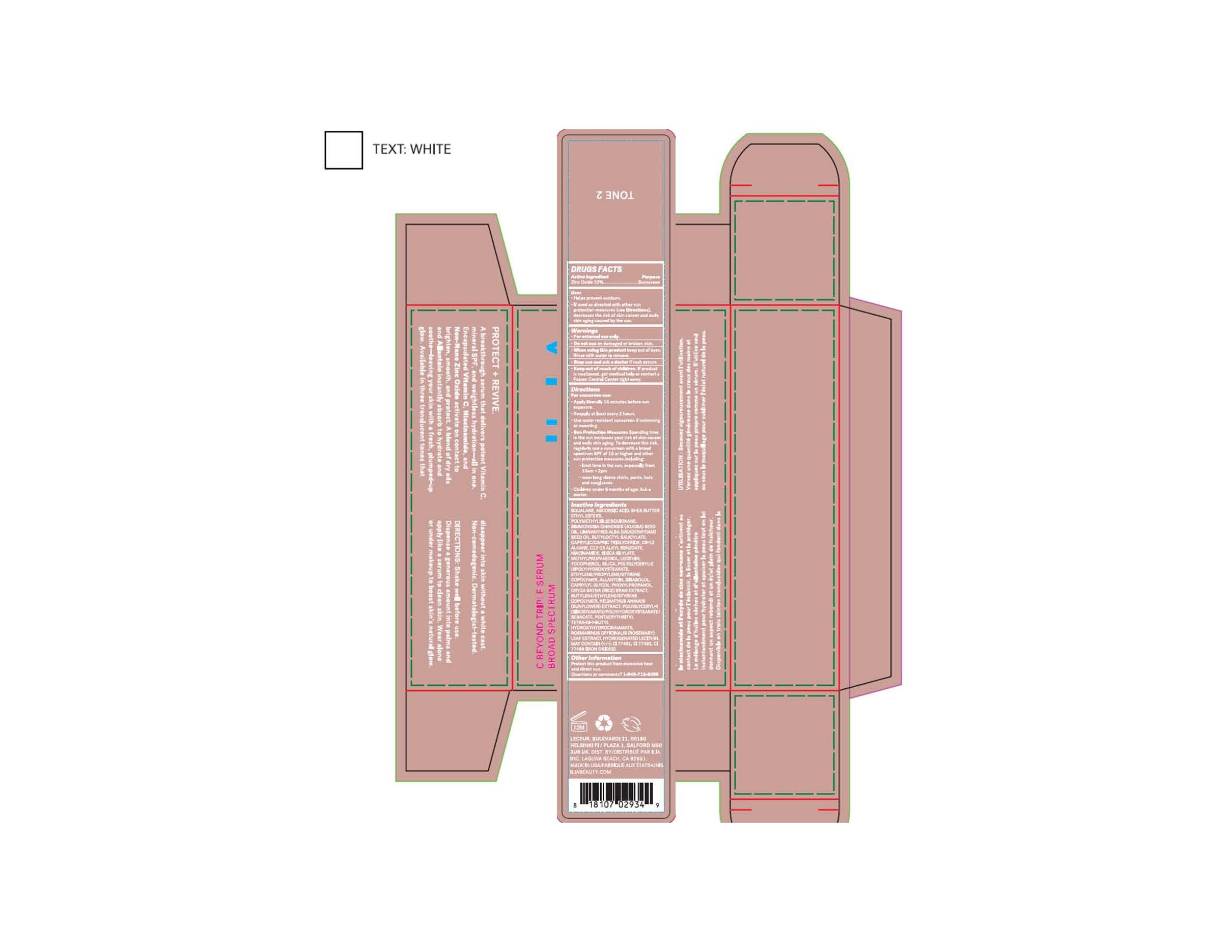
C BEYOND TRIPLE SERUM (TONE 2)
C BEYOND TRIPLE SERUM (TONE 2) by
Drug Labeling and Warnings
C BEYOND TRIPLE SERUM (TONE 2) by is a Otc medication manufactured, distributed, or labeled by Ilia, Inc., Nanophase Technologies Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
C BEYOND TRIPLE SERUM (TONE 2)- zinc oxide lotionÂ
Ilia, Inc.
----------
C BEYOND TRIPLE SERUM (TONE 2)
| C BEYOND TRIPLE SERUM (TONE 2)Â
zinc oxide lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler -Â Ilia, Inc. (078503090) |
| Registrant -Â Nanophase Technologies Corporation (623502044) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Nanophase Technologies Corporation | 050383046 | api manufacture(81110-071) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Nanophase Technologies Corporation | 118812921 | pack(81110-071) , manufacture(81110-071) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Nanophase Technologies Corporation | 623502044 | api manufacture(81110-071) , manufacture(81110-071) | |
Revised: 3/2025
Â
Document Id: 2ffe3639-a253-cfeb-e063-6394a90a3da8
Set id: dac27057-13d0-efd5-e053-2a95a90a038e
Version: 6
Effective Time: 20250310
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.