Antimicrobial Hand Sanitizer Spray Fresh Scent
Antimicrobial Hand Sanitizer by
Drug Labeling and Warnings
Antimicrobial Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by HPC Ventures, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
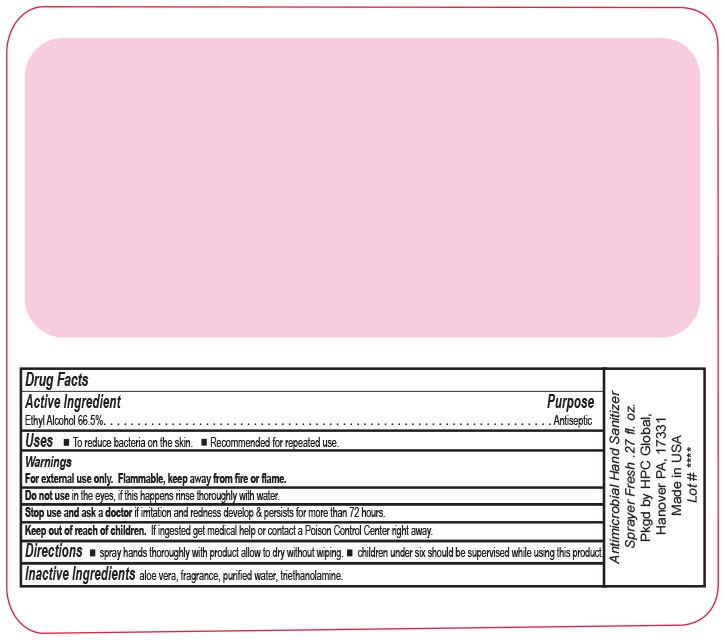
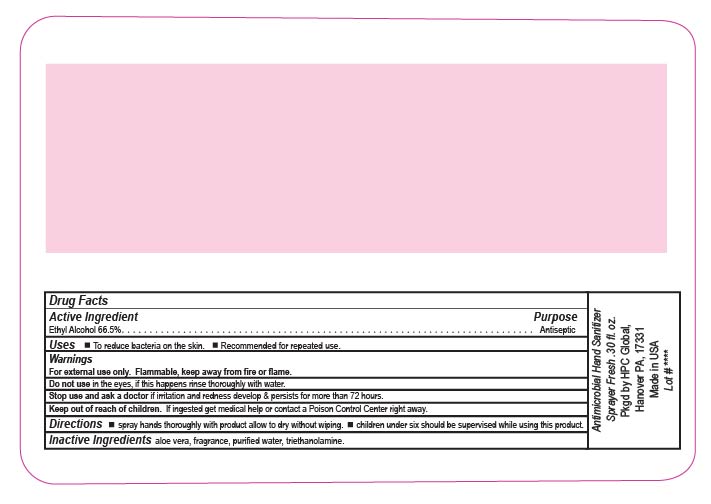
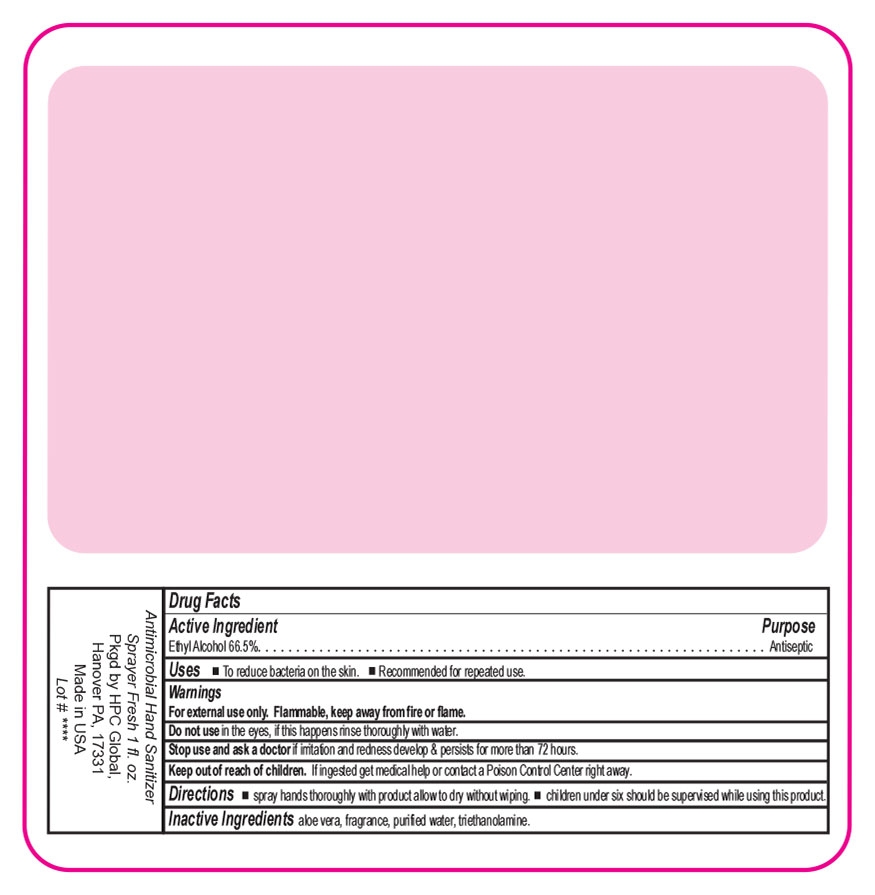
ANTIMICROBIAL HAND SANITIZER- alcohol liquid
HPC Ventures, LLC
----------
Antimicrobial Hand Sanitizer Spray
Fresh Scent
Keep out of reach of children. If ingested get medical help or contact a Poison Control Center right away.
Directions wet hands thoroughly with product allow to dry without wiping children under six should be supervised while using this product.
| ANTIMICROBIAL HAND SANITIZER
alcohol liquid |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - HPC Ventures, LLC (097538103) |
Revised: 6/2025
Document Id: 3c434344-667e-eeee-e063-6294a90a8f2a
Set id: dae79bca-bb73-af47-e053-2a95a90ae587
Version: 3
Effective Time: 20250627
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.