MAG-AL PLUS XS- aluminum hydroxide, magnesium hydroxide, and dimethicone suspension
Mag-AL Plus by
Drug Labeling and Warnings
Mag-AL Plus by is a Otc medication manufactured, distributed, or labeled by Pharmaceutical Associates, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses for the relief of:
-
Warnings
Do not take more than 8 teaspoonfuls in a 24-hour period or use the maximum dosage for more than 2 weeks except under the advice and supervision of a physician.
- Directions
-
Other information
- each 5 mL contains: magnesium 167 mg, sodium 2.24 mg
- store at controlled room temperature 20° - 25°C (68° - 77°F) [see USP Controlled Room Temperature].
- protect from freezing
- White colored, cherry flavored liquid supplied in the following oral dosage form:
NDC: 0121-1762-30: 30 mL unit dose cup. Case contains 100 unit dose cups of 30 mL packaged in 10 trays of 10 unit dose cups each.
*Maalox is a registered trademark of Novartis Consumer Health, Inc.
- Inactive ingredients
- Questions or comments? Call 1-800-845-8210
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 30 mL Cup Tray Label
* Compare to the
active ingredients
in Maalox ® Max™NDC: 0121-1762-30
MAG-AL PLUS XS
Maximum Strength Antacid/ Anti-Gas
Sugar FreeEach 30 mL contains:
Magnesium Hydroxide 2400 mg
Aluminum Hydroxide 2400 mg
Simethicone 240 mgSHAKE WELL
USUAL DOSAGE: See attached Drug Facts
This unit-dose package is not child-resistant.
Store at 20° - 25°C (68° - 77°F)
[See USP Controlled Room Temperature].
Protect from freezing.10 x 30 mL Unit-Dose Cups
Pharmaceutical
Associates, Inc.
Greenville, SC 29605T1762300519
R05/19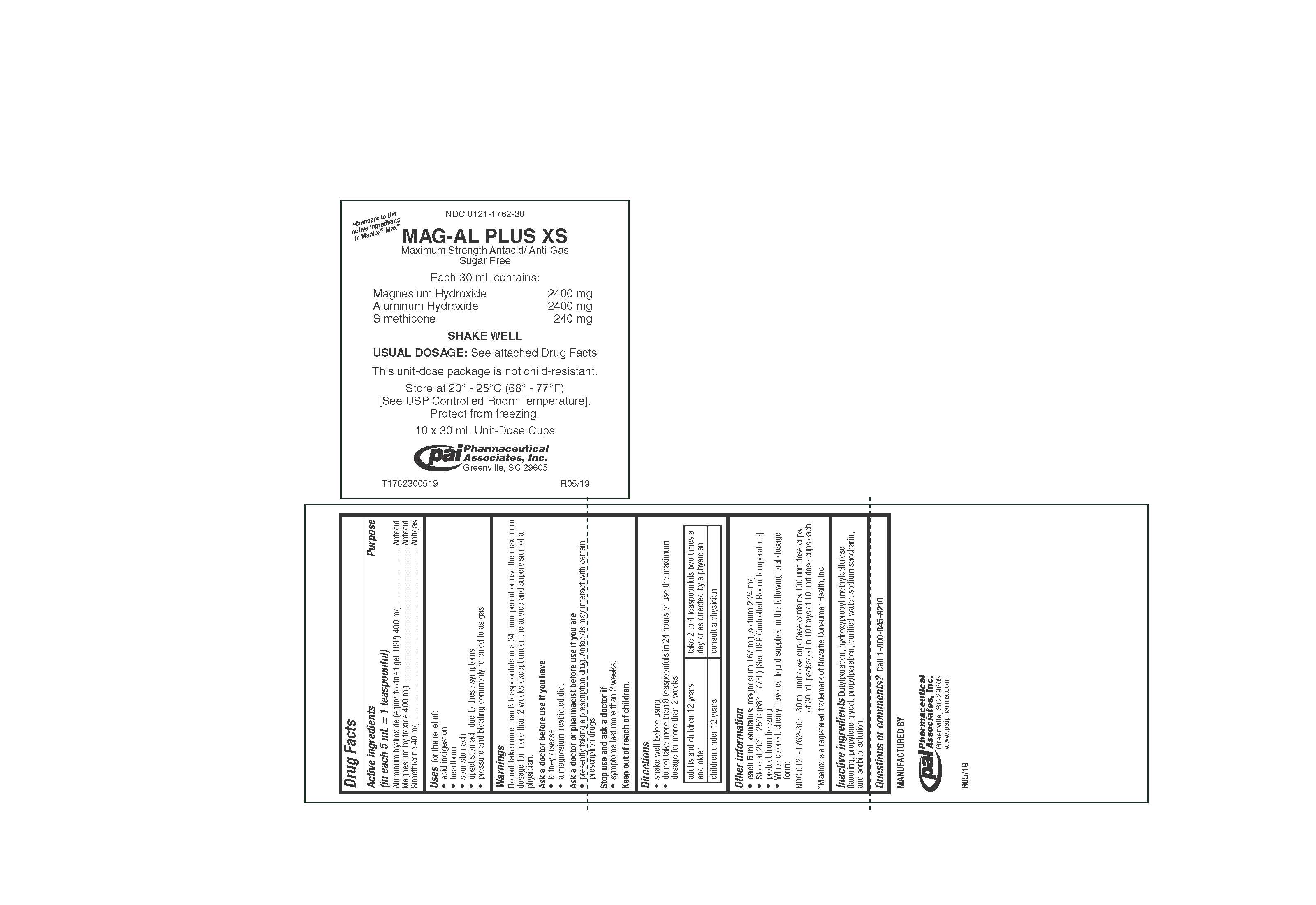
-
INGREDIENTS AND APPEARANCE
MAG-AL PLUS XS
aluminum hydroxide, magnesium hydroxide, and dimethicone suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0121-1762 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) (ALUMINUM HYDROXIDE - UNII:5QB0T2IUN0) ALUMINUM HYDROXIDE 400 mg in 5 mL MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) (MAGNESIUM CATION - UNII:T6V3LHY838, HYDROXIDE ION - UNII:9159UV381P) MAGNESIUM HYDROXIDE 400 mg in 5 mL DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 40 mg in 5 mL Inactive Ingredients Ingredient Name Strength BUTYLPARABEN (UNII: 3QPI1U3FV8) PROPYLPARABEN (UNII: Z8IX2SC1OH) HYPROMELLOSE 2910 (4000 MPA.S) (UNII: RN3152OP35) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SORBITOL (UNII: 506T60A25R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) PEPPERMINT OIL (UNII: AV092KU4JH) CYCLOMETHICONE 4 (UNII: CZ227117JE) WATER (UNII: 059QF0KO0R) Product Characteristics Color white Score Shape Size Flavor PEPPERMINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0121-1762-30 10 in 1 CASE 01/14/2004 1 10 in 1 TRAY 1 30 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 01/14/2004 Labeler - Pharmaceutical Associates, Inc. (044940096) Establishment Name Address ID/FEI Business Operations Pharmaceutical Associates, Inc. 097630693 manufacture(0121-1762)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.