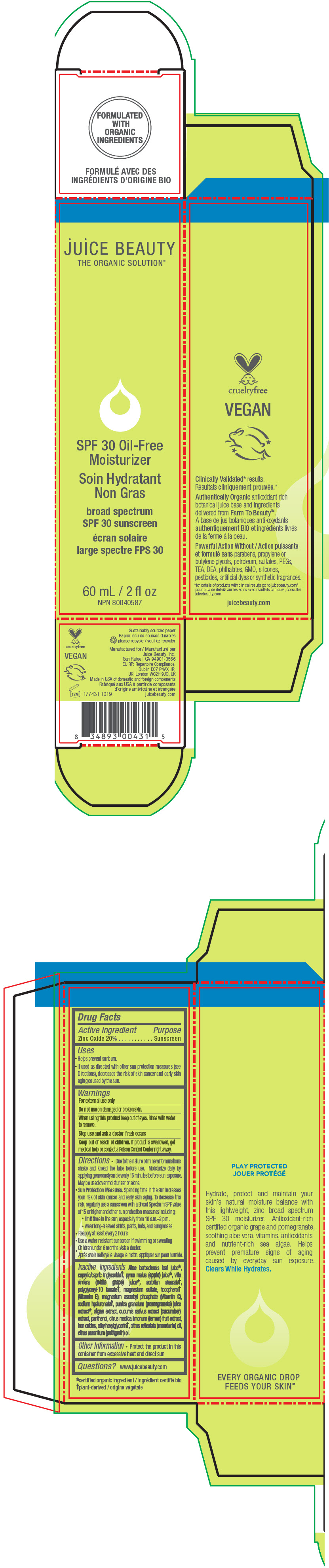
SPF 30 OIL-FREE MOISTURIZER- zinc oxide lotion
SPF 30 Oil-Free Moisturizer by
Drug Labeling and Warnings
SPF 30 Oil-Free Moisturizer by is a Otc medication manufactured, distributed, or labeled by Juice Beauty. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
-
Uses
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- Due to the nature of mineral formulations shake and knead the tube before use. Moisturize daily by applying generously and evenly 15 minutes before sun exposure. May be used over moisturizer or alone.
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.–2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- Reapply at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating
- Children under 6 months: Ask a doctor.
Après avoir nettoyé le visage le matin, appliquer sur peau humide.
-
Inactive Ingredients
Aloe barbadensis leaf juice1, caprylic/capric triglyceride2, pentylene glycol2, polyglyceryl-6 distearate2, glyceryl stearate2, sorbitan sesquioleate2, pyrus malus (apple) juice1, vitis vinifera (grape) juice2, ascorbyl glucoside (Vitamin C), hyaluronic acid, bisabolol2, punica granatum (pomegranate) pericarp extract2, punica granatum (pomegranate) extract2, laminaria digitata (sea kelp) extract1, pongamia pinnata seed extract2, cucumis sativus (cucumber) fruit extract1, citrus limon (lemon) fruit extract2, glycerin2, xanthan gum, polyhydroxystearic acid2, sodium stearoyl glutamate, polyglyceryl-6 polyricinoleate2, maltodextrin2, citric acid, silica, iron oxides (CI 77491, CI 77492, CI 77499), jojoba esters2, simmondsia chinensis (jojoba) seed oil2.
- 1 certified organic ingredient
- 2 plant-derived
- Other Information
- Questions?
- PRINCIPAL DISPLAY PANEL - 60 mL Tube Carton
-
INGREDIENTS AND APPEARANCE
SPF 30 OIL-FREE MOISTURIZER
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 55165-0202 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 20 g in 100 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) pentylene glycol (UNII: 50C1307PZG) polyglyceryl-6 distearate (UNII: Z35I17EQOP) GLYCERYL 1-STEARATE (UNII: 258491E1RZ) sorbitan sesquioleate (UNII: 0W8RRI5W5A) WINE GRAPE JUICE (UNII: JHQ6158A7R) apple juice (UNII: 9871T0PD5P) ASCORBYL GLUCOSIDE (UNII: 2V52R0NHXW) hyaluronic acid (UNII: S270N0TRQY) LEVOMENOL (UNII: 24WE03BX2T) POMEGRANATE FRUIT RIND (UNII: RS999V57DU) POMEGRANATE (UNII: 56687D1Z4D) LAMINARIA DIGITATA (UNII: 15E7C67EE8) PONGAMIA PINNATA SEED (UNII: C2BRV53B1V) CUCUMBER (UNII: YY7C30VXJT) LEMON (UNII: 24RS0A988O) glycerin (UNII: PDC6A3C0OX) xanthan gum (UNII: TTV12P4NEE) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) sodium stearoyl glutamate (UNII: 65A9F4P024) polyglyceryl-6 polyricinoleate (UNII: YPM0ZOC2HR) maltodextrin (UNII: 7CVR7L4A2D) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) JOJOBA OIL, RANDOMIZED (UNII: 7F0EV20QYL) JOJOBA OIL (UNII: 724GKU717M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55165-0202-1 60 mL in 1 TUBE; Type 0: Not a Combination Product 05/15/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 05/15/2010 Labeler - Juice Beauty (263151582)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.